More Information
Submitted: November 07, 2025 | Approved: November 28, 2025 | Published: December 01, 2025
How to cite this article: Onwujiogu VC, Orjiocha SI, Chinonso EF, Salem MAS, Bhat AS, Mehandi R, et al. Chromatography Hyphenated Techniques for the Analysis of Natural Products (A Review). Arch Case Rep. 2025; 9(12): 383-406. Available from:
https://dx.doi.org/10.29328/journal.acr.1001177
DOI: 10.29328/journal.acr.1001177
Copyright license: © 2025 Onwujiogu VC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hyphenated chromatography techniques; Natural products analysis; GC–MS; HPLC–MS; LC–FTIR; LC–NMR–MS; UHPLC–MS; Ion Mobility Spectrometry (IMS); Structural elucidation; Metabolite profiling
Chromatography Hyphenated Techniques for the Analysis of Natural Products (A Review)
Vivian Chinekwu Onwujiogu1*, Samuel Ibezim Orjiocha2, Eze Faith Chinonso1, Mansour AS Salem3, Aadil Shafi Bhat4, Rabiya Mehandi5 and Abugu Hillary Onyeka1
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Enugu State, Nigeria
2Department of Science Laboratory Technology, University of Nigeria, Nsukka, Enugu State, Nigeria
3Department of Chemistry, University of Aden, Aden, Yemen
4Department of Chemistry, Aligarh Muslim University, Uttar Pradesh, India
5Department of Chemistry, Jamia Millia Islamia, New Delhi, India
*Address for Correspondence: Vivian Chinekwu Onwujiogu, Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Enugu State, Nigeria, Email: [email protected]
Hyphenated techniques for natural product analysis significantly enhance the separation and identification of complex mixtures. Among these, Gas Chromatography-Mass Spectrometry (GC-MS) excels at analyzing volatile and semi-volatile compounds. Food safety, forensics, medicines, environmental analysis, and other fields depend on it heavily, and new technical developments are always improving its abilities and extending its uses. Sensitivity and selectivity of GC-MS have greatly increased in recent years. Automated sample preparation methods improve efficiency, decrease human error, and streamline operations. Machine learning techniques also improve data analysis by allowing automated peak detection, quantification, and compound property prediction. High-throughput analysis is facilitated by ultra-high-performance liquid chromatography (UHPLC) and high-performance liquid chromatography-mass spectrometry (HPLC-MS). When it comes to locating and measuring the bioactive substances in medicinal plants, HPLC-MS is quite useful. Liquid Chromatography-Fourier Transform Infrared Spectroscopy (LC-FTIR) provides complementary benefits by combining liquid chromatography’s separation capabilities with FTIR’s structural elucidation. LC-FTIR improves molecular composition comprehension and quantitative capabilities, as chemometrics advances. Combining LC-FTIR with additional methods, such as LC-MS, offers a full perspective of sample composition. Along with these technological advancements, green chromatography methods such as eco-friendly solvents, supercritical fluid chromatography (SFC), energy-efficient apparatus, and low-waste sample preparation are gaining popularity for decreasing the ecological impact. This study aims at understanding the principles, advantages, limits, scientific evaluation, applications and differences in modern hyphenated chromatographic technique which have found relevance in natural products analysis.
Chromatography hyphenated techniques belong to a family of analytical methodologies that integrate the potential to separate with spectroscopic detection technologies offering structural elucidation [1,2]. Natural products continue to make up a significant fraction of the more than 0.5–1 million known organic compounds since they have been a key source of compounds with medicinal or nutritional value for generations [3]. An exponential growth of secondary metabolites is thought to occur, and many of these remain undiscovered. In the past 20 years, high throughput screening and combinatorial chemistry have emerged, which has led to a decrease in natural product research despite its significance [4]. The complexity of chemical analysis, especially in natural product research promoted the development of new analytical techniques. Chromatography-hyphenated techniques proved to be a breakthrough over the years, providing unsurpassed specificity and sensitivity [5].
Chromatography was first used by the Russian botanist Mikhail Tswett in 1903, who divided plant pigments with calcium carbonate and coined the word chromatography [6]. This work was the basis for chromatographic principles of separation by selective affinity. Although paper chromatography and thin-layer chromatography (TLC) were primitive, they made important contributions to separation science in the mid-20th century with their ability to easily separate complex mixtures. The emergence of gas chromatography (GC) in the 1950s, and high-performance liquid chromatography (HPLC) in the 1960s kick-started an age of accuracy in separation science [7].
Some advanced chromatographic techniques include High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Affinity Chromatography, Ion Exchange Chromatography, and Size Exclusion Chromatography Ghandi, et al. 2022. The choice of chromatography technique depends on the properties of the analytes and the desired level of purity [8].
The hyphenated technique is a term that was introduced to define the combination of chromatographic separation with one or more spectroscopic detectors [9]. The motivation for this innovation stems from the necessity of a highly detailed characterization of chromatographically separated entities which was unattainable with traditional detection systems [10]. Hyphenated techniques have evolved with the advancement of technology. These methods have benefited from the miniaturization of components, advanced detector technologies, and computer-aided data analysis, which allow the valid and reliable analysis of more complex mixtures with great accuracy and precision [11]. The analysis of natural products usually involves complex mixtures of chemical compounds present at micro-concentration levels [12]. Separation of the different compounds present in a given natural product is the first analytical step required for the subsequent isolation and purification of the various components of interest, especially when chemically complex natural products are being studied [13].
Over the years, hyphenated techniques have revolutionized natural products analysis allowing unprecedented discovery of new compounds with more complex structures and improved knowledge of their intricate biological functions [14]. These techniques are essential in fields such as pharmacognosy, metabolomics, toxicology, quality control & assurance, environmental analysis, and much more [15]. This review paper deals with advances and possibilities in modern chromatography techniques used in natural product analysis.
Fundamentals and basic principles of chromatography
For the purpose of this review, chromatography is an investigative method in which the original ingredients are slightly changed to appear on an analytical column at various periods or locations [16]. A solvent (for example, organic solvents are frequently used in liquid chromatography), the gas phase, supercritical fluids (such as “SFC”), ion-pair solutions, micellar solutions, or even chromatography done directly on a support (such as solid-phase microextraction) can all be used as the mobile phase [17]. It is preferable to think of the chromatographic process as a two-stage procedure. The materials that need to be separated are first divided into the mobile and stationary phases. The substance to be separated is first divided into the stationary and mobile phases. The components of the sample are then carried along a percolating path—which is essentially an extended, thin sponge-like region—by a particular force caused by the movement of the mobile phase [18]. Time determines how the surface area that an analyte is adsorbed onto; this dependence on time is caused by analyte diffusion. In other words, since the solvent preferentially flows down the column in the direction of the detector, the analytes must first migrate from the sample counter-diffuse to the solvent and be able to seep through the porous media [19]. Phase balance is crucial as it determines how an analytical column function. To prevent interstitial capillaries, columns are filled with uniform particles; for example, a peak should be Gaussian to enable the equilibrium target distribution to be desorbed isothermally together with its absorptive fossil [20]. Reproducibility from one batch to another batch is equally crucial. When columns are packed so that mass transmission is the same between each column, equivalency across sheets is observed. That is to say that the diameter, particle size, and column packing all affect the mass transfer across the column [21]. The above stepwise flow pattern is determined by measuring and investigating the sources of solute retention. Below are key fundamentals of chromatographic techniques.
1. The equilibrium partitioning of an analyte between the mobile and stationary phases is described by the distribution, often known as the partition coefficient (K). Maintaining a constant K is crucial for reproducible separations [22].
2. Ensuring that, in order to prevent peak distortion from nonlinear influences, the separation takes place inside the linear area of the adsorption isotherm [23].
3. Maximizing solute diffusion rates and minimizing linear velocity to approach local equilibrium between the phases [24].
4. The use of different stationary phase chemistries (e.g. normal phase, reversed phase, ion exchange, and affinity) to selectively retain analytes based on their properties [25].
5. High pressures and small stationary phase particles are used in techniques like GC and HPLC to provide highly effective and high-resolution separations [26].
The following are parameters involved in chromatographic analysis [27].
Retention Time (tR): The time it takes for a component to pass through the system from the point of injection to detection.
Selectivity Factor (α): This is the ratio of the retention factors of two components. This is presented in Equation 1.
(1) [28] where 𝑘2 and 𝑘1 represent the retention factors of the two components.
Retention factor (k): Indicates how much time a chemical is in the mobile phase as opposed to the stationary phase. Calculated as in Equation 2.
(2) [29]
where 𝑡 is the retention time of the compound, and 𝑡0 is the time it takes for the unretained solute to pass through the system.
Resolution (R): This is the measure for the degree of separation between two components. It is determined by Equation 3.
(3) [30]
where 𝑡𝑅1 & 𝑡𝑅2 are the retention times of the two components, and 𝑤1 & 𝑤2 are the widths of the peaks at baseline.
Hyphenated techniques, advantages, limitations and their applications in natural product analysis
According to Awuchi and Twinomuhwezi [1], Chromatography hyphenated techniques commonly used for the analysis of natural products include:
1. Gas Chromatography-Mass Spectrometry (GC-MS)
The process begins with sample injection and culminates in the data system, comprising a computer and software, is vital for collecting, processing, and analyzing the data generated by the GC-MS [31] (Figure 1).
Figure 1: GC – MS Representation [32].
This technique is widely used in various fields, including environmental analysis, pharmaceuticals, food safety, and forensics [33].
Continuous advancements in GC-MS technology have expanded its applications and enhanced its performance, combining the separation power of gas chromatography (GC) with the molecular identification capabilities of mass spectrometry (MS). This combination allowed for the detailed analysis of volatile and semi-volatile compounds, revolutionizing the field of analytical chemistry. The coupling of GC to MS provided orthogonal selectivity chromatographic retention plus mass spectral fragmentation which significantly improved both qualitative and quantitative accuracy [34]. These dual-selection mechanisms minimized false positives and helped establish GC-MS as the “gold standard” for forensic toxicology, pesticide residue analysis, environmental pollutant detection, and natural product profiling [35]. The introduction of tandem mass spectrometry (MS-MS) and time-of-flight (TOF) detectors has broadened the scope of GC-MS applications, particularly in environmental chemistry [35].
Capillary gas chromatography has gained broad acceptance and application in the analysis of organic compounds, including those with complex compositions. This trust is founded on a number of laws [35]. 1) In comparing conditions in planar chromatography with atmospheric distillation, the separational capability of gas chromatography (GC) methods under optimal separation conditions can be provided with an efficiency level of 1012 equivalent theoretical plates [36]. This efficiency is achieved by the use of narrow-bore capillary columns covered with thin stationary phase films that reduce band widening and improve resolution. The separation process is governed by the partition coefficient (K), which determines how compounds are distributed between the stationary and mobile phases. According to Aguilar Meza, et al. [37], molecules with a higher affinity for the mobile phase elute faster, whereas those with a higher affinity for the stationary phase have longer retention times.
2) The ability of this method to separate decomposition products without decomposing the analytes provides almost non-existent probability for co-elution [38]. 3) Sample interaction with column material can also be minimized. Although being of infrequent occurrence, the design of a column with a film thickness equating to just a few micrometers can roughly determine the qualitative characteristic of certain hexadecyl poly (dialkylsiloxane) fillers, which boast thin films, pervaporation, and a specific level of phase domain formation characteristics [39]. 4) For the most part, the situation of substance groups giving categorical chromatography separates extremely well to the point where it is mostly reduced to simple optimization calibration analysis method, as well as efficiency being reduced to a set performance [40]. 5) This method also allows separation of single isomers for highly complex organic substance groups with longer separation time [41].
Recent advancements in GC-MS have resulted in enhanced sensitivity and selectivity. New ionization techniques and mass analyzers have been developed to improve detection limits and selectivity. High-resolution mass spectrometry (HRMS) has become prevalent, utilizing TOF and Orbitrap analyzers for high-resolution and accurate mass measurements, thereby enabling better identification of compounds [42].
Comprehensive two-dimensional gas chromatography (GC×GC) represents another significant advancement. By coupling two GC columns with different stationary phases, this technique provides improved separation of complex mixtures [43]. Automated sample preparation systems have also been integrated into the workflow, streamlining sample extraction, purification, and derivatization processes, which reduces human error and increases efficiency [44].
Data analysis and machine learning have revolutionized the processing of GC-MS data. Advanced software and machine learning algorithms are now used for data processing, peak identification, and quantification. These tools also predict compound properties, enhancing the accuracy and reliability of the analysis [45].
Satyal [46] used GC-MS to determine the chemical makeup of essential oils isolated from different plants. The study demonstrated the capacity of GC-MS to detect and quantify main and minor components in complicated mixtures. The study successfully illustrates the excellent resolution and sensitivity of GC-MS in separating and detecting volatile chemicals.
Hu, et al. [47] used GC-MS to profile metabolites in medicinal plants, revealing a diverse spectrum of bioactive chemicals. The study underscored the technique’s efficacy in uncovering novel natural compounds with potential medicinal applications. While the work demonstrates the flexibility of GC-MS in natural product identification, it also highlights the limitations of GC-MS in evaluating non-volatile, thermally unstable chemicals. Further study shows that the analysis can be enhanced by coupling GC-MS with derivatization techniques such as high-resolution mass spectrometry (HRMS) or tandem mass spectrometry (MS/MS).
Lee, et al. [48] investigated how environmental conditions impact plant metabolomic patterns using GC-MS. Changes in temperature and soil conditions caused considerable variances in secondary metabolites, according to the researchers. The study sheds light on how the environment changes the composition of natural products.
Patel [49] and his team used GC-MS to detect volatile chemicals in traditional herbal treatments and associate them with their medicinal capabilities. The investigation verified the existence of numerous known bioactive chemicals and discovered novel ones. This work highlights the importance of GC-MS in natural product research, particularly in the context of traditional medicine.
Garcia-Valverde, et al. [50] explored the application of comprehensive two-dimensional gas chromatography coupled with mass spectrometry (GCxGC-MS) to analyze complex natural product mixtures. The approach provided enhanced separation and improved identification of compounds in samples with a wide range of chemical diversity. The use of GCxGC-MS represents a significant improvement in the field, addressing the challenge of co-elution in one-dimensional GC-MS.
The advantages of GC-MS are numerous. From providing trace analysis where GC-MS excels at detecting and quantifying compounds present at very low concentrations, to early compound detection due to its sensitivity, which is crucial for applications such as disease diagnosis, food safety, and quality control [51]. GC-MS also provides unique/highly specific fingerprints, thereby minimizing the risk of false positives or negatives, which is attributed to the mass spectra generated by GC-MS [52].
GC-MS can distinguish between isomers and structurally similar compounds, making it ideal for analyzing complex mixtures [53].
By comparing experimental mass spectra to spectral libraries, researchers can identify unknown compounds, which can help in drug discovery, environmental research, and forensic investigations Brown, et al. 2020.
GC-MS equally offers precise and accurate quantification of analytes through calibration curves and internal standards, also the combination of chromatographic separation and mass spectral data provides robust data for quality control and regulatory compliance [51].
In the analysis of natural products, GC-MS is preferred over HPLC-MS and UHPLC-MS when analyzing volatile and semi-volatile natural products e.g., essential oils, terpenes, alkaloids with low polarity, and when high chromatographic resolution is needed for small, thermally stable compounds [54,55]. GC-MS is also preferred when extensive spectral libraries for compound identification are required [55].
Like every other man-made thing, GC-MS has some limitations which stem from the physicochemical properties of natural compounds, technical constraints of the technique and the complexity of natural matrices.
1. GC-MS is limited to the analysis of volatile and thermally stable compounds. Natural products such as high-molecular-weight polysaccharides, proteins, and thermally labile compounds (e.g., certain alkaloids and glycosides), cannot be subjected to GC-MS analysis without derivatization. Derivatization, also known as chemical structure modification [56], is the process of chemically altering a compound to enhance its volatility, thermal stability, or detectability. This is made possible by adding a functional group or modifying already existing ones, making the compound more suitable for GC-MS analysis. Common derivatization reactions include silylation, acylation, alkylation, and esterification [57].
Derivatization, although useful, complicates sample preparation and may degrade sensitive compounds [38,58].
2. Thermally labile compounds can decompose at the high temperatures required for GC separation, resulting in false identification and quantification. For example, some flavonoids and terpenoids degrade easily under GC conditions, resulting in the loss of structural integrity and the formation of decomposition products that interfere with data interpretation [52].
3. Polar compounds, like organic acids, sugars, and amino acids are not suited for GC-MS analysis due to their low volatility and strong interactions with the stationary phase [59].
4. Complex mixtures of compounds are often found in natural product extracts with similar physicochemical properties, which can cause co-elution and overlapping peaks in GC-MS chromatograms [14].
5. Though GC-MS offers molecular weight and fragmentation patterns, it offers limited structural information compared to techniques like NMR or LC-MS/MS. For instance, distinguishing between isomers or resolving complex stereochemistry can be challenging with just GC-MS, necessitating the use of complementary techniques for complete structural characterization [53].
6. Sample preparation for GC-MS analysis, especially for natural products, can be laborious, time consuming and require major cleanup to remove interfering matrix components. Solid-phase extraction (SPE) and liquid-liquid extraction (LLE) are commonly used extraction procedures, but these methods can lead to the loss of target analytes or contamination [44].
7. Matrix effects, such as ion suppression or amplification, can significantly affect the accuracy and precision of GC-MS data. Natural product matrices, which often contain high levels of co-extractives like pigments and lipids, can interfere with ionization and detection, leading to biased quantification [51].
Because of a number of significant technological advancements that significantly improved the precision of chemical analysis, GC-MS is regarded as revolutionary in analytical chemistry [55]. With the introduction of electron-ionization (EI) and chemical-ionization (CI) sources, reproducible fragmentation patterns that could be utilized to create large mass spectral libraries were made possible, making GC-MS the first method that could quickly and accurately identify unknown compounds [60,61].
Also, the switch from packed columns to high-efficiency capillary columns, which increased separation efficiency by several orders of magnitude, was another significant development [62]. Under ideal circumstances, their theoretical plate counts (up to 10¹²) significantly decreased co-elution, enabling GC-MS to resolve complex mixtures that were previously impossible to separate using conventional GC or TLC techniques [63] (Figure 2).
Figure 2: Schematic diagram showing analytical packing system showing major components required for high efficiency packing [62].
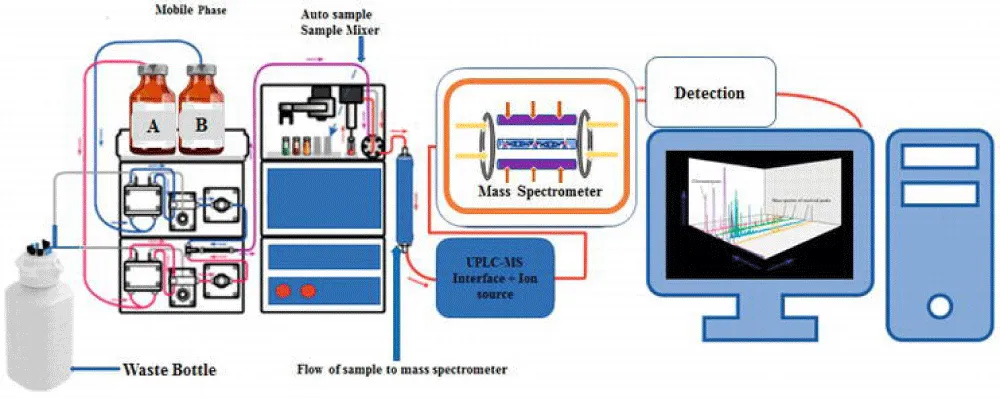
2. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS)
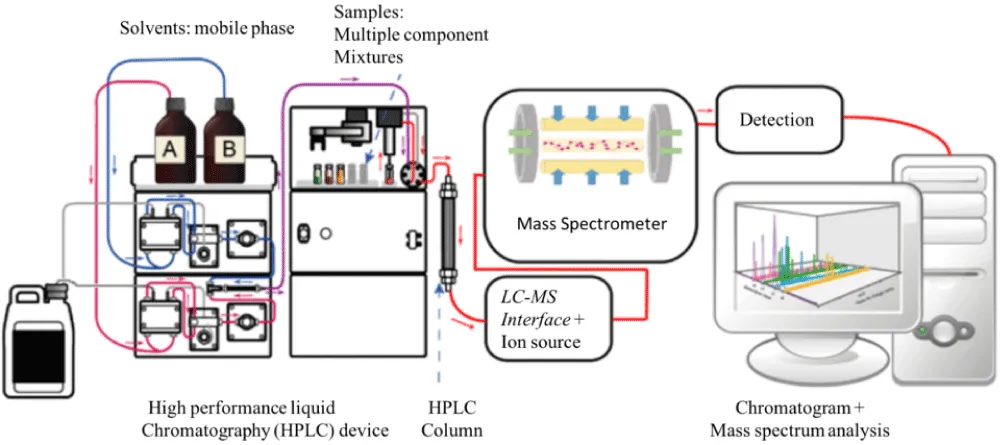
HPLC-MS is a standard technology for both researchers and industry experts, providing uncommon capabilities to analyze complex mixtures [64]. It is a versatile instrument that can handle a wide range of chemicals, from small molecules like pharmaceuticals to big macromolecules like proteins, making it useful in fields such as drug development, environmental studies, and food science [65]. HPLC separates chemicals based on their interactions with the stationary and mobile phase, enabling the separation, identification and quantification of individual components in complex samples [25]. Modern MS detectors, particularly tandem MS (MS/MS), are extremely sensitive, allowing the identification of trace-level analytes even in challenging matrices [66]. MS extracts structural information from molecules based on their fragmentation patterns. This is critical for identifying new compounds and understanding their characteristics. The technique has become more accessible to a larger variety of academics and aiding high-throughput analysis with the development of smaller, more automated HPLC-MS equipment [2] (Figure 3).
Figure 3: Flow Representation of HPLC-MS [67].
By pushing the limits of separation speed and resolution, Ultra-High-Performance Liquid Chromatography (UHPLC) technology enables even quicker analysis times and greater peak capacity for complex samples [68]. Mass spectrometry has advanced recently, providing excellent mass accuracy and resolution, direct sample analysis without prior preparation, workflow simplification, and increased applicability of HPLC-MS, all of which contribute to more reliable compound identification and isomer differentiation [2].
HPLC-MS is widely used in the analysis of medicinal plants to identify and quantify bioactive compounds such as alkaloids, flavonoids, terpenes, and acids [69]. The technique provides detailed insights into the chemical composition of plant extracts, thereby aiding the discovery of new therapeutic agents [70].
In recent years, the development of green chromatography methods has gained attention in natural products analysis. Researchers are increasingly focused on minimizing the environmental impact of analytical methods [71]. Innovations such as supercritical fluid chromatography (SFC) and the use of green solvents in HPLC-MS provide more sustainable alternatives while maintaining high analytical performance [72]. These environmentally friendly techniques support the principles of green chemistry and sustainability, promoting environmentally responsible research practices [73]. Due to the inherent complexity of bioanalytical difficulties, high-performance liquid chromatography-mass spectrometry (HPLC-MS), frequently referred to as the “gold standard” of bioanalysis, has survived more than 30 years of continuous technology innovation and process advancements [74]. The continuous improvement of HPLC-MS, which pushes the boundaries of science by measuring numerous biomolecules in difficult and diverse matrices, is linked to overcoming matrix interference and improving assay selectivity, accuracy, sensitivity, robustness, and assay speed [75]. Industry-setting assay requirements in the low pg/mL/performance liquid chromatography-tandem mass spectrometry multiple reaction monitoring (LC-MS/MS MRM) range through the administration of biologically relevant mixtures have made it even more difficult to monitor exogenous drugs, their metabolites, and endogenous biomarkers via HPLC-MS at sub-pg/mL to pg/mL levels in complex matrices [76]. Liquid chromatographic columns, gradient behavior, mobile phases and mobile phase additives, ionization techniques, mass analyzers, and acquisition modes are only a few of the areas of HPLC-MS theory and practice where advances have been made due to uncompromising demands in technological requirements [77]. The Strengths and Advantages associated with the use of HPLC-MS in natural products analysis include:
Enhanced sensitivity: Trace levels of bioactive substances can be detected by modern MS detectors, especially tandem MS (MS/MS), which is essential for researching the distribution and possible bioactivity of these compounds [78].
Unparalleled Power for Complex Mixtures: Natural products often consist of complex mixtures of several substances. These may be separated according to polarity with great success using HPLC-MS, enabling individual characterization [11,79].
Structural elucidation: MS fragmentation patterns provide helpful insights regarding the structure of a molecule. This is helpful with the identification of unidentified natural products, a common problem in this sector [2].
Both qualitative and quantitative analyses are possible with HPLC-MS because of its dual functionality, which can be used to determine both “how much” and “what’s there” in terms of information. Understanding the chemical makeup of natural products and their possible therapeutic benefits is crucial [80].
Data rich in information: A multitude of data regarding mass, retention duration, and fragmentation are produced by HPLC-MS [44].
In a recent study, Smith, et al. [81] used HPLC-MS to identify and quantify bioactive chemicals in medicinal plants. To evaluate extracts of Panax ginseng, they used UHPLC in conjunction with a quadrupole time-of-flight (QTOF) mass spectrometer. The main bioactive ingredients of Panax ginseng, approximately 50 ginsenosides, were effectively discovered by the study. Accurate molecular weight and structural identification of these substances was made possible by the use of high-resolution mass spectrometry (HRMS). Significant differences in ginsenoside content were found by quantitative investigation across various plant sections and development phases.
Likewise, Jones, et al. [82] used a thorough HPLC-MS technique, combining reversed-phase chromatography with an Orbitrap mass spectrometer, in research to characterize the metabolome of the marine species. The investigation used both focused and non-focused metabolomics to offer a comprehensive understanding of the chemical makeup of the sponge. In the conclusion, the study demonstrated the efficacy of HPLC-MS in thorough metabolomic profiling in addition to highlighting the existence of new compounds with possible medicinal benefits. Accurate mass measurements and high-resolution detection made possible by the use of an Orbitrap MS are crucial for untargeted analysis.
In another study, Lee, et al. [83] used HPLC-MS to evaluate the phytochemical composition of many dietary supplements. The samples were examined by utilizing a triple quadrupole mass spectrometer in conjunction with HPLC. Multiple reaction monitoring (MRM) was used in the investigation to accurately quantify the desired phytochemicals. Significant differences in the phytochemical composition of several brands of dietary supplements were found by the investigation. Important bioactive substances, including flavonoids and polyphenols, were also found, and their amounts were measured in the study.
HPLC-MS and UHPLC-MS are preferred techniques when analyzing non-volatile, thermally labile, or polar natural products like flavonoids, alkaloids, and glycosides [84]. Both techniques are also preferred in cases where there is no need for derivatization (as required in GC-MS for non-volatile compounds), and when higher sensitivity is required for trace-level detection [79].
The limitations of HPLC-MS are similar to those of UHPLC-MS. HPLC-MS requires expensive instrumentation and frequent maintenance. It also requires careful optimization of mobile phase, ionization conditions, and mass analyzer selection [85]. Complex sample matrices can interfere with ionization, affecting accuracy and reproducibility.
HPLC-MS provides molecular weight but requires additional techniques like MS and NMR for full structural analysis [86]. Additionally, Poor ionization of non-polar or neutral compounds may necessitate derivatization, increasing complexity [87].
3. Liquid Chromatography-Fourier Transform Infrared Spectroscopy (LC-FTIR)
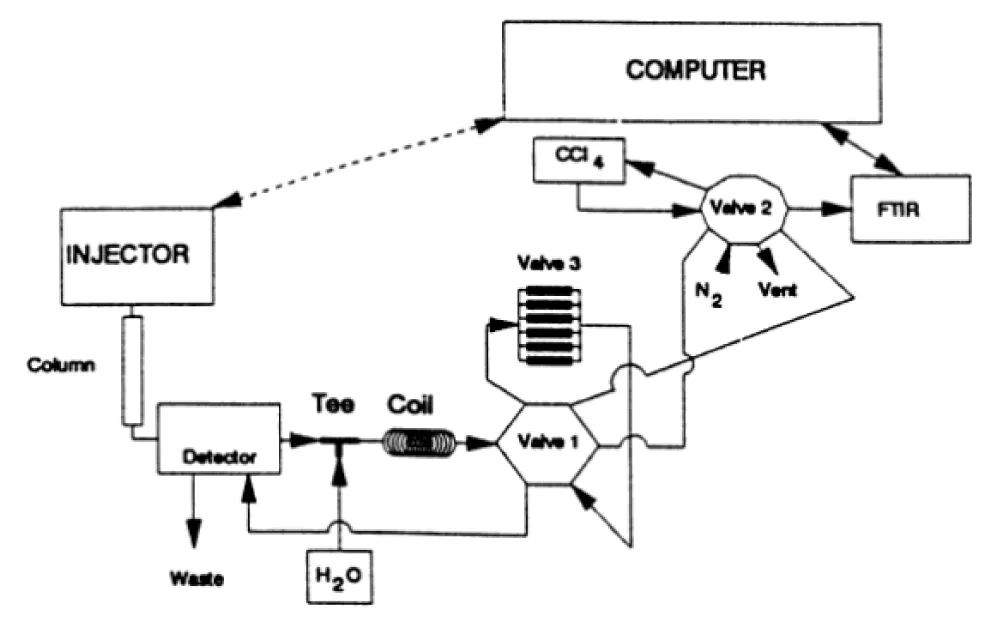
An effective analytical method that has been widely applied in the study of natural products is liquid chromatography-Fourier transform infrared spectroscopy [88]. The integration of liquid chromatography’s separation skills with Fourier transform infrared spectroscopy’s extensive structural elucidation and identification capabilities results in this coupling [89]. Since the technique enables the simultaneous separation and structural characterization of the different components, LC-FTIR is especially helpful for the investigation of complex mixtures, such as those seen in natural product extracts [90]. The molecular composition and structure of the substances present are ascertained using FTIR spectroscopy, which is based on the sample’s absorption of infrared light [91] (Figure 4).
Figure 4: Flow layout of LC-FTIR [92].
The quantitative capabilities of FTIR spectroscopy have been further improved by recent developments in chemometrics and software algorithms, enabling the precise and trustworthy measurement of natural product ingredients [93]. This has been shown to be quite helpful in fields like biomedicine and pharmaceuticals, where accurate measurement of active ingredients is crucial [94].
Chemists who work on the identification and biosynthesis of secondary metabolites have been paying more and more attention to natural products [95]. Particularly, compounds such as lignins, terpenoids, and flavonoids are significant in how plants react to a variety of stresses, such as pathogenic and physical assaults. Their research has started to reveal comparable functions for a few of the essential oils [96].
Typically, chemists have combined thin-layer chromatography (TLC) with sensitive chemical or biochemical examinations. These procedures require a lot of effort and time, and in certain cases, they depend on feeding radioactive precursors [97]. For example, it has long been hypothesized that meillonin biosynthesis targets the enzyme system that directly generates the 3-hydroxy-3-methylglutaryl intermediate from acetate; this idea has recently been thoroughly validated [98]. The development of Fourier Transform Infrared Spectroscopy (FTIR) has made it feasible to study biological extracts without the need for sensitive staining methods or radioactive precursors. Since infrared spectroscopy (IR) is a destructive method, the only way to link the flow of the sample to the result is by comparing the relevant chromatograms [99]. It is now feasible to directly connect the infrared (IR) of the product to discrete parts of the chromatogram by tying liquid chromatography (LC) to this extremely precise detection technology. This method provides a number of strong benefits for the analysis of complex samples [100].
The ability of LC to separate complicated mixtures is a major benefit. Analysis can be hampered when certain components of a mixture are frequently obscured by others. By dividing the mixture according to particular physicochemical characteristics, LC solves this difficulty [101]. This enables the separate FTIR examination of every component, guaranteeing a more thorough comprehension of the sample’s makeup. Besides separation, LC-FTIR also provides insightful information on the molecular structure of the separated components [77]. The ability of FTIR spectroscopy to disclose a molecule’s functional groups is excellent. By serving as a kind of fingerprint, this data makes it easier to identify components that are unknown and gives us more insight into their chemical makeup [102].
The complementary character of LC-FTIR in relation to other analytical techniques is another benefit [103]. While methods such as LC-MS are excellent at identifying particular compounds, LC-FTIR provides a more comprehensive view of the functional groups that are present. Because of their complementary nature, researchers may make use of each technique’s advantages to provide a more complete view of a sample’s composition [104]. In addition, LC-FTIR can often be non-destructive. After analysis, the sample is frequently not altered. This provides opportunities for additional research or even the possible recovery of the separated components for use in subsequent analyses, which is a big benefit for rare or valuable materials [105].
Liquid chromatography was used by Zhang, et al. [106] to isolate the constituents of essential oils, and FTIR spectroscopy was then utilized for structural analysis. Important ingredients in the essential oils, including linalool and menthol, were effectively identified by the study. FTIR spectra confirmed the existence of several phenolic compounds and terpenes by providing precise information on functional groups. The results of comparative research showed that the chemical makeup of oils from various regions varied.
Zhang’s research demonstrated how well LC-FTIR performs when assessing complicated mixtures, such as essential oils. Precise identification and structural clarification of constituents were made possible by the combination of infrared spectroscopy and chromatography.
Kim, et al. [107] separated and analyzed the polyphenols in green tea using high-performance liquid chromatography (HPLC) coupled with FTIR. A thorough spectrum analysis was part of the investigation to pinpoint important functional groups. Major polyphenols, including epicatechins and catechins, as well as their derivatives, were found by the investigation. FTIR spectra shed light on the carbonyl and hydroxyl groups that define polyphenols. The work demonstrated how spectral fingerprints may be used by LC-FTIR to differentiate between different polyphenolic substances. Kim, et al. skillfully illustrated how to use LC-FTIR for polyphenol profiling, highlighting the method’s potential to yield structural details. Although the spectral analysis of the study was extensive, quantitative information on the concentration of polyphenols was absent. Quantitative LC-MS analysis might improve the results and present a more comprehensive picture.
Gonzalez, et al. [23] employed LC-FTIR to examine herbal supplement samples that were suspected of being adulterated in a related investigation. The goal of the study was to identify medicinal compounds that were not reported and synthetic additives. Several undisclosed substances, such as steroids and synthetic stimulants, were found in the herbal supplements, according to the research. FTIR spectra showed distinctive peaks that matched the adulterant’s functional groups. The study highlighted the possible health hazards linked to contaminated herbal items. Gonzalez demonstrated the value of LC-FTIR in assuring product safety by offering important insights into the identification of adulterants in herbal supplements. The capacity of the research to recognize a wide variety of adulterants is one of its strongest points.
Additionally, Singh, et al. [108] isolated alkaloids from plant extracts using liquid chromatography and then employed FTIR analysis to characterize the structural features of the mixture. The study concentrated on medicinal plants rich in alkaloids, including Rauvolfia serpentina and Catharanthus roseus. The investigation found vincristine and reserpine to be two important alkaloids. FTIR spectra confirmed the existence of quinoline and indole alkaloids by providing extensive information on the functional groups. Based on these alkaloids’ structural properties, the study emphasized the medicinal uses they may have. Singh, et al. successfully characterized alkaloids using LC-FTIR, offering insightful information on their structural makeup. Although the study’s comprehensive spectrum analysis was impressive, it lacked quantitative information on the quantities of alkaloids. Combining LC-FTIR with quantitative methods like HPLC-UV or LC-MS might help future studies give a more thorough understanding.
For functional group identification, LC-FTIR is preferred to LC-NMR-MS, for instance, when distinguishing between isomers according to carbonyl or hydroxyl functional groups [109]. LC-FTIR is equally preferable when characterizing new compounds in plant extracts without the use of spectral libraries and when the sample needs to be preserved for further testing or when non-destructive analysis is required [110].
LC-FTIR has some limitations that can make other techniques preferred for natural products analysis. The limitations are as followsIn aqueous-based LC separations, the identification of analytes may be hampered by the high infrared absorption bands of water. On account of this, IR-transparent solvents or post-column solvent removal methods are required [111].
While FTIR offers useful functional group information, it is not as capable as methods such as LC-MS or LC-NMR for elucidating high-resolution molecular structures. Compound identification may become less certain as a result [112].
The overlapping IR absorption bands of complex mixtures can make spectral interpretation challenging, requiring advanced software and spectral libraries for accurate analysis [113].
FTIR detectors need specialized optics and attenuated total reflectance (ATR) accessories, which increase the system’s complexity and cost in contrast to traditional UV or MS detectors [114].
A good number of LC-FTIR systems require post-column solvent evaporation or solvent exchange before IR detection to minimize solvent interference. This extra step may increase analysis time and reduce throughput [77].
4. Liquid Chromatography-Nuclear Magnetic Resonance Spectroscopy-Mass Spectrometry (LC-NMR-MS)
This recently developed hyphenated technique combines the sensitivity and molecular weight information from MS with the separation power of LC and the structural elucidation capabilities of NMR [112]. The use of the analytical approach in the field of natural products has been documented recently, owing to the exceptional benefit of LC-NMR-MS. Complex mixtures must first be divided into their parts according to their respective physicochemical characteristics, such as polarity and affinity for the stationary phase, using liquid chromatography (LC) [115]. The separated components enter the NMR spectrometer when they elute from the LC column. This is where the molecules’ detailed structural information is found, which offers important details regarding connectivity, stereochemistry, and functional groups [116]. The mass spectrometer, which measures the compounds’ molecular weight, receives the LC effluent as well. Furthermore, MS fragmentation patterns can supplement NMR with additional structural information [117].
There are various benefits associated with LC-NMR-MS. This hyphenated method totally provides quantitative and structural information for compound characterization [112]. It can be difficult to differentiate between isomers using conventional methods, but LC-NMR-MS does a great job at it [112]. In contrast to some other analytical techniques, LC-NMR-MS is often non-destructive, enabling further analysis or sample recovery [118]. This method works especially well for examining complex mixtures, such as natural products, where it might be difficult to distinguish several components that have similar characteristics [119].
The use of LC-NMR-MS in the examination of natural products is revolutionary [120]. Complex mixtures may now be quickly characterized by researchers without the need for time-consuming separation and purification procedures [121]. This effectiveness makes it possible to find compounds that could otherwise go unnoticed [14]. Furthermore, the method’s capacity to distinguish between isomers—which present major challenges for conventional analytical techniques—is crucial in the field of natural product chemistry [119].
Researchers like Tan, et al. [122] used LC-NMR-MS to comprehensively profile secondary metabolites in herbal extracts. While molecular weight information and further structural insights were obtained by MS, NMR was utilized to get precise structural information. Numerous secondary metabolites, including flavonoids, alkaloids, and terpenoids, were effectively discovered by the study. The NMR spectra provided comprehensive structural details, including stereochemistry and connectivity, whereas the MS verified the molecular weights and fragmentation patterns. The combination of methods made it possible to identify a number of unique compounds that had not been documented before in any literature.
LC was used by Wang, et al. [123] to isolate the antioxidant components found in fruit extracts. While MS revealed fragmentation patterns and molecular weight information, NMR provided structural information. Many antioxidants, such as vitamin C, flavonoids, and polyphenolic substances, were found in the research. NMR spectra provided comprehensive structural details, such as stereochemistry and functional groups. The identification of the antioxidants was aided by the confirmation of the molecular weights and fragmentation patterns by MS data. A thorough comprehension of the antioxidant chemicals was made possible by the combination of approaches.
To isolate the complex mixture of bioactive chemicals present in marine algal preparations, Kim, et al. [124] employed liquid chromatography (LC). Molecular weight information and fragmentation patterns were provided by MS to assist in compound identification, while NMR supplied comprehensive structural details. For the integration of LC, NMR, and MS data, the study offered a clear process. Polysaccharides, peptides, and small-molecule metabolites were among the bioactive substances that the investigation also found. The three-dimensional structures and functional groups of the compounds that were found were described in detail by the NMR spectra. Clarifying the structure was made easier by the confirmation of fragmentation patterns and molecular weights by MS data.
Singh, et al. [125] also used LC-NMR-MS to analyze phytochemicals in traditional medicinal plants. Numerous phytochemicals were found in the study, such as glycosides, phenolic acids, and flavonoids. While MS data validated the molecular weights and fragmentation patterns, supporting the identification procedure, NMR spectra offered comprehensive structural information, including stereochemistry and connectivity.
LC-NMR-MS is preferred over LC-MS when full structural elucidation is required, especially for novel compounds without spectral library matches [126]. It is also favored in cases where differentiating positional isomers that have identical mass spectra is necessary and in analyzing complex mixtures where compounds co-elute in LC-MS, making mass spectra alone insufficient [127].
In spite of its many advantages, LC-NMR-MS has some limitations. The technique requires sophisticated instrumentation and expertise, which can be expensive and time-consuming [128]. Also, NMR’s sensitivity is generally lower than MS, which can limit its use for trace analysis [129]. However, in recent years, NMR sensitivity has greatly improved due to advancements in cryogenic technology and flow probes [130].
5. Ultra-High-Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS)
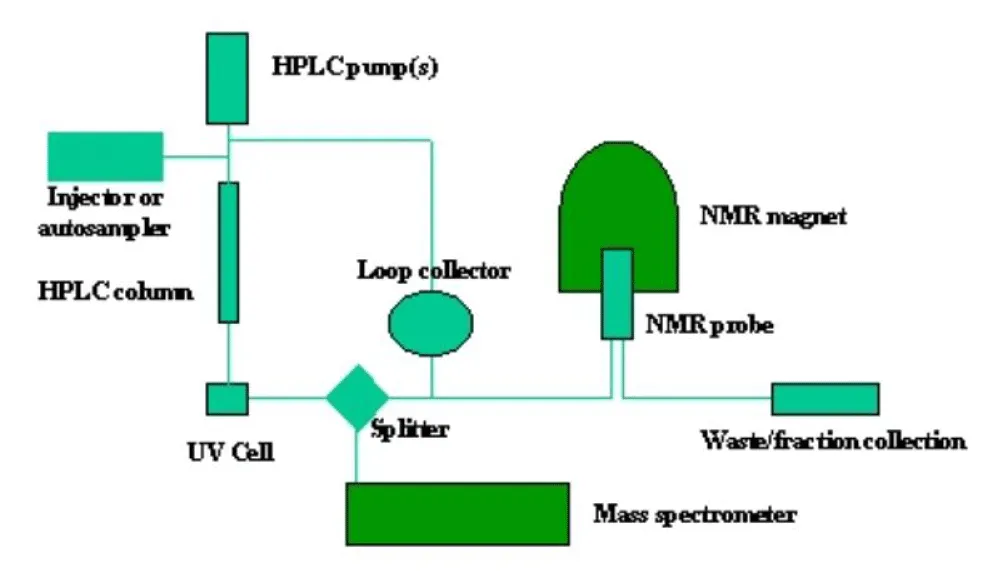
UHPLC operates on the same fundamental principles as High-Performance Liquid Chromatography (HPLC) but utilizes smaller particle sizes (typically sub-2 µm) in its stationary phase [131]. This is crucial for operating at greater pressures (up to 100 MPa), which will lead to quicker and more effective separations [132]. By utilizing mass spectrometry integration, molecules may be identified and quantified according to their mass-to-charge ratio, yielding comprehensive insights into the chemical composition and structure of the analytes [2]. Although both UHPLC and HPLC use comparable operating procedures, UHPLC offers superior chemical separation because of smaller particle sizes and considerably shorter analytical times Nahar, et al. 2020. Figure 5 presents the flow diagram for UHPLC-MS.
Figure 5: Flow Representation of LC-NMR-MS [133].
The capacity of UHPLC-MS to handle a wide variety of analytes is a unique benefit. This method shows remarkable adaptability in handling small molecules as well as biomacromolecules [134]. Due to its sensitivity, it can identify and measure molecules at the trace level. Additionally, high-throughput analysis is made possible by UHPLC-MS’s speed, which speeds up the screening and quality control processes [135]. Other advantages include: Improved Resolution and Sensitivity: The instrument can identify and measure minute components in intricate matrices because of UPLC’s much better peak resolution and sensitivity, particularly caused by its smaller particle size columns and higher working pressures [136] (Figure 6).
Figure 6: Flow diagram of UHPLC-MS [137].
Quick analysis: UHPLC-MS provides significantly quicker analysis times than conventional HPLC-MS, thereby boosting the volume of samples and effectiveness [138].
Better data quality: Cleaner spectra and more accurate data are produced by the combination of sensitive mass detection and high-resolution separations, which makes compound identification and quantification easier Guo, et al. 2020.
In a study by Chen, et al. [139], researchers looked into the chemical composition of traditional Chinese medicinal herbs using UHPLC-MS. Identifying and quantifying the bioactive substances that give these plants their medicinal properties was the main goal. The MS component supplied comprehensive molecular information, such as molecular weights and fragmentation patterns, whereas the UPLC component was employed for its effectiveness in separating complex mixtures. They pointed out that compounds present at low concentrations that could have gone undetected with less sophisticated methods have been found thanks to the excellent sensitivity and resolution of UHPLC-MS. The study emphasized the use of UPLC-MS in the thorough examination of medicinal plants, which aids in the identification of possible novel therapeutics. Chen, et al. did, however, draw attention to the necessity of additional in vitro and in vivo research to confirm the biological activities of the discovered compounds and support their therapeutic potential.
Similarly, Johnson, et al. [140] utilized UHPLC-MS to examine marine sponges in particular and other secondary metabolites in marine species. The objective of the study was to investigate the chemical variety of marine sponges and find substances that could have therapeutic uses. The exact mass analysis and effective separation of the metabolites were made possible using the UHPLC-MS technology. A number of new polyketides and peptides with noteworthy bioactivity, such as antibacterial and anticancer capabilities, were discovered by Johnson, et al. Precise molecular weight measurements and the ability to differentiate between structurally identical molecules were made possible by the high-resolution capabilities of UHPLC-MS. The study brought attention to the marine sponges’ unrealized potential as a source of new bioactive chemicals.
In another recent study, García, et al. [141] utilized UHPLC-MS to investigate the polyphenolic content of various fruit extracts. The research aimed to identify and quantify polyphenolic compounds, which are known for their antioxidant properties. The use of UHPLC-MS enabled the separation of complex polyphenolic mixtures and provided accurate mass data for compound identification. García, et al. identified a wide range of polyphenols, including flavonoids, phenolic acids, and tannins, and quantified their concentrations in different fruit extracts. The researchers highlighted the health benefits associated with these polyphenols, such as reducing the risk of chronic diseases due to their antioxidant activity. The study demonstrated the effectiveness of UHPLC-MS in the detailed profiling of polyphenolic compounds, contributing valuable information to the field of nutritional science.
UHPLC-MS is preferred over HPLC-MS when there is a need for faster analysis with better resolution and peak capacity. Also, when there is limited sample volume, a need for high-throughput screening of complex plant extracts, and efficiency in solvent usage is important, UHPLC is the technique of choice [142].
The major limitations of UHPLC-MS are underlisted.UHPLC-MS systems are expensive to purchase and maintain, requiring specialized columns, high-pressure pumps, and advanced mass spectrometers. Operational costs, including ultra-pure solvents and maintenance, are also higher compared to traditional HPLC-MS [143,144].
Optimizing UHPLC-MS methods is time-consuming and requires experience. High pressures and rapid flow rates demand careful adjustment of parameters like column chemistry, particle size, and gradient conditions [52].
UHPLC requires specialized columns with small particle sizes (<2 µm), which are more susceptible to clogging and degradation. Older HPLC columns are often incompatible, limiting flexibility [145].
High-resolution UHPLC-MS data is complex to interpret and generates large file sizes, requiring advanced software and significant storage capacity. This can complicate data management and analysis [52].
UHPLC-MS is prone to matrix effects, such as ion suppression or enhancement, which can affect quantification [146].
6. Liquid Chromatography- Ultra Violet- Mass Spectrometry (LC-UV-MS)
This technique combines the separation capabilities of liquid chromatography with the detection and quantification abilities of ultraviolet (UV) and mass spectrometry (MS) for the separation and identification of complex mixtures [112]. The combination of LC-UV with MS provides a comprehensive approach to understanding the structural and kinetic characteristics of natural products. LC separates compounds based on their chemical properties, while UV provides a preliminary identification based on absorbance, and MS offers detailed molecular information [147]. This combination is effective for detecting and characterizing small molecules in complex mixtures [148].
LC-UV-MS is widely applied in various natural product analyses, including:
Metabolite profiling: Identification of secondary metabolites such as alkaloids, flavonoids, and terpenoids in medicinal plants [149].
Quality control of herbal medicines: Standardization and authentication of herbal formulations based on characteristic UV and MS fingerprints [150].
Pharmacokinetic studies: Determination of bioavailability and metabolic pathways of natural products in biological samples [151].
Food and nutraceutical analysis: Detection of bioactive polyphenols, carotenoids, and vitamins in food products [152].
Advantages of LC-UV-MS abound, and a few of them are underlined.
LC-UV-MS offers a robust method for initial screening and quantification of compounds with UV-active chromophores. It is widely used due to its simplicity and cost-effectiveness.
LC-UV-MS is versatile as it is suitable for a wide range of natural products, including polar and non-polar compounds.
This technique is highly Sensitive as the MS enhances detection limits compared to UV alone.
The non-destructive UV Detection allows for real-time monitoring of chromatographic elution.
Limitations of LC-UV-MS [112,151,153,154]
- LC-UV-MS is limited by its reliance on UV-active compounds, which may not be present in all natural products. It cannot differentiate isomers effectively
- Non-chromophoric compounds like some terpenoids remain undetected by UV.
- The combined UV and MS spectra require advanced spectral deconvolution techniques.
- Co-eluting compounds may affect UV absorbance and ionization efficiency in MS due to matrix interference.
- Matrix effects may lead to variable ionization efficiency, affecting quantification accuracy.
Advancements in LC-UV-MS aim to enhance analytical capabilities in the areas of improved UV detectors with higher sensitivity, High-Resolution Mass Spectrometry (HRMS), miniaturization of LC-UV-MS systems for field analysis, and integration with Artificial Intelligence for automated peak identification and data processing [155].
LC-UV-MS is preferred over stand-alone UV or MS techniques for its ability to provide both qualitative and quantitative data in a single run [156]. It is also preferred when a combination of UV and MS detection can enhance compound characterization, especially for UV-active compounds such as polyphenols, carotenoids, and flavonoids [157]. LC-UV-MS is equally the ideal technique when rapid screening of samples is needed using UV fingerprints [158].
In a recent study by Saidi, et al. [159], the phytochemical composition, biological activities, and pharmacological properties of Ajuga iva (L.) leaf extracts were investigated to confirm its traditional use in Moroccan ethnomedicine for treating diabetes, microbial infections, and oxidative stress. The study focused on analyzing the plant’s chemical composition, antioxidant, antimicrobial, and antidiabetic properties using various in vitro and in vivo methods.
In the study, LC-UV-MS played a crucial role in the phytochemical analysis of Ajuga iva by identifying and characterizing its polyphenolic compounds. Specifically, the aqueous extract of Ajuga iva was analyzed using LC-UV-MS, which led to the identification of 32 polyphenolic compounds, including ferulic acid (19.06%), quercetin (10.19%), and coumaric acid (9.63%) and apigenin-7-(2-O-apiosylglucoside) (6.8%). LC-UV-MS provided a comprehensive chemical profile, which correlated with the plant’s antioxidant, antimicrobial, and antidiabetic activities, demonstrating that LC-UV-MS is a powerful tool for metabolite profiling in natural product research.
In another study by Tinnirello, et al. [160], the protective effects of industrially produced lemon-derived nanovesicles (iLNVs) in inflammatory bowel disease (IBD) using a rat model were analysed. The key findings in the study were a) Characterization of iLNVs, b) In Vitro Studies (on Human Macrophages), c) in vivo Effects (Rat Model of Colitis).
Qualitative and quantitative characterization of iLNV metabolites was carried out. Qualitative analysis of iLNVs was performed by UHPLC-UV-ESI-MS. Q-Exactive Plus mass spectrometer equipped with an electrospray source (ESI) and coupled to an Ultimate 3000 SD UHPLC. Citric acid and isocitric acid content were also quantified by UHPLC-UV-ESI-MS, consisting of a Q-Exactive Plus mass spectrometer equipped with an electrospray source (ESI) and coupled to an Ultimate 3000 SD UHPLC. This step provided a comprehensive chemical profile of LNVs, highlighting the specific compounds responsible for their therapeutic effects. It also helped support the study’s findings on the antioxidant activity of LNVs and their role in reducing oxidative stress in colitis.
7. LC-Ion-Mobility/LC-Ion-Mobility-MS
For this review, liquid chromatography ion mobility spectrometry (LC-Ion-Mobility) and liquid chromatography ion mobility mass spectrometry (LC-Ion-Mobility-MS) will be discussed together.
Liquid Chromatography-Ion Mobility Spectrometry (LC-IMS) integrates liquid chromatography (LC) with ion mobility spectrometry (IMS) to enhance the separation and analysis of complex mixtures, especially in the study of natural products [161]. This combination offers enhanced resolution and structural elucidation capabilities by utilizing the unique separation processes of both approaches [68].
In LC-IMS, liquid chromatography is used to first separate substances according to their physicochemical characteristics. After that, the eluted chemicals are ionized and put into the IMS cell, where an electric field separates the ions according to how easily they can move through a neutral gas. Finally, the separated ions are fed into the mass spectrometer, where they undergo additional analysis based on their mass-to-charge ratio, allowing for structural elucidation and precise mass determination [162]. Several variables, including the ion’s size, shape, charge, and collision cross-section, affect its mobility. Ions that are smaller or more compact usually go through the IMS cell more quickly than those that are longer or bigger [163]. Combining LC and IMS improves the study of complicated mixtures by offering a multidimensional separation strategy [164].
In natural product research, LC-IM-MS helps to characterize bioactive substances with structural similarities. Many natural products have isomeric or identical structures, complicating analysis [165]. The inclusion of ion mobility makes it possible to distinguish between these compounds, making it easier to identify and structurally elucidate novel bioactive molecules with pharmacological or nutraceutical potential [78].
A key advantage of LC-IMS is its enhanced separation efficiency. The combination of LC and IMS provides orthogonal separation processes; this means that compounds are separated according to both their chromatographic retention time and gas-phase ion mobility [166]. This makes it possible to resolve compounds that could otherwise co-elute in conventional LC methods, leading to improved identification and quantification of complex mixtures [167].
Another benefit is the ability of IMS to provide structural information about analytes. The IMS component provides insights into the gas-phase conformations of ions, which is particularly useful for distinguishing between isomers and conformers [168]. This structural differentiation is crucial in applications such as natural product analysis and metabolomics, where many compounds share similar molecular weights but differ in their three-dimensional structure [169].
LC-IMS also allows for quick analysis, as IMS operates on a millisecond timescale. This fast separation capability enables high-throughput screening and real-time analysis, making the approach appropriate for applications requiring fast and efficient sample processing [123]. The speed of IMS does not affect the quality of the results, ensuring both rapid data capture and high-resolution separation [170].
Also, LC-IMS improves sensitivity by increasing the signal-to-noise ratio. The additional ion mobility separation stage reduces chemical noise, resulting in better detection of low-abundance compounds. This benefit is especially useful in trace analysis, where detecting minute amounts of bioactive chemicals can be difficult [171]. With the inclusion of mass spectrometry MS, the system provides superior evaluation of complex samples by utilizing different separation mechanisms [166].
Limitations of LC-Ion Mobility Spectrometry/LC-Ion-Mobility-MS
1. Differences in ionization efficiencies between compounds can adversely affect the sensitivity and quantitative accuracy of the assay [172].
2. The multidimensional data generated require advanced computational tools and skills for proper interpretation [173].
3. Integrating IMS with LC and mass spectrometry (MS) adds to the system’s complexity, potentially increasing maintenance requirements and operational costs [174].
4. The integration of LC, IMS, and MS results in a high-cost, complex instrument requiring regular calibration and maintenance [175].
5. The multidimensional data generated in LC-IMS-MS requires advanced bioinformatics tools for interpretation [176].
6. Unlike traditional MS databases, IMS spectral libraries are still under development, which makes the identification of unknown compounds more challenging [177].
LC-IMS is particularly preferred in cases where separation based on molecular shape, size, and charge is the primary objective [178]. Since IMS differentiates molecules based on their gas-phase mobility, it is especially useful for resolving isomeric compounds that may exhibit similar mass spectra but differ in structural conformation [179]. This makes LC-IMS a valuable instrument for researching compounds with small structural differences that typical LC techniques may find difficult to separate [166].
The integration of mass spectrometry and LC-IMS increases analytical power, making it the preferred approach in more complex studies [166]. LC-IMS-MS is especially useful for evaluating complex natural product mixtures containing isobaric or structurally related compounds, as it adds a dimension of separation beyond mass-to-charge ratio alone [180]. Furthermore, where the investigation requires separation based on gas-phase conformation, LC-IMS-MS increases resolution and aids in the identification of compounds that would otherwise be indistinguishable [181]. This approach is also very useful for studying conformational differences in biomolecules like peptides and flavonoid glycosides, where structural variations play an important role in biological function and activity [182].
In a study by Venter, et al. 2018, the power of combining multiple chromatographic and spectrometric techniques for comprehensive chemical analysis was leveraged.
This approach greatly enhanced the resolution of complex phenolic mixtures. HILIC successfully separated highly polar phenolic compounds, but RP-LC proved more effective for non-polar and moderately polar compounds. IMS provided an additional layer of separation based on molecule size, shape, and charge to help differentiate structurally related substances.
By taking advantage of the orthogonality of these approaches, the study produced greater phenolic compound characterization, allowing for better distinction of isomers and structurally similar species. The use of IMS assisted in resolving co-eluting substances that would otherwise be indistinguishable in conventional LC-MS analysis.
In another article by Zheng, et al. [183], the application of modern analytical techniques to characterize the chemical composition of diverse portions of the medicinal plant Gynostemma longipes was investigated. The authors used two-dimensional liquid chromatography (2D-LC) combined with ion mobility-mass spectrometry (IM-MS) to accomplish high-resolution separation and identification of multicomponent mixtures in the plant. The study discovered a variety of bioactive substances, including saponins, flavonoids, and phenolic acids, which are renowned for their therapeutic qualities. The use of 2D-LC-IM-MS revealed its potential to do thorough multicomponent analysis, paving the way for future medicinal plant studies.
Carnevale Neto, et al. [184], in a study, investigated the use of ion mobility spectrometry (IMS) in the investigation of complex natural product mixtures. The study looked at how IMS, paired with mass spectrometry (MS), can improve the structural characterization and constitutional assignment of compounds in natural product extracts. The study emphasized the limitations of evaluating complex mixtures, such as overlapping peaks and structural similarities, and showed how IMS can increase resolution and provide new structural insights. The key findings in the study are summarized below:
Ion mobility spectrometry (IMS), which adds a dimension of separation based on size, shape, and charge, greatly enhances the separation of chemicals in complex natural product mixtures. As a result, the resolution of structurally related molecules is improved, and peak overlap is decreased. The study shows how well IMS-MS analyzes complex natural product extracts, including those from microbes and plants. It highlights how IMS can distinguish between isomers and structurally similar molecules, which are frequently difficult to resolve with traditional MS alone.
Current trends and future directions
Current trends and prospects in chromatography hyphenated techniques reflect significant advancements in analytical chemistry, particularly in enhancing the sensitivity, speed, and resolution of complex sample analyses [164,185]. These techniques combine chromatographic processes with spectroscopic detection to offer both comprehensive qualitative and quantitative data. Here’s an overview of the existing climate and anticipated developments in this field [186] (Tables 1,2).
| Table 1: Table comparing the different chromatography hyphenated techniques [187,188,189]. (Kumar & Bogusz, 2021; Queiroz, et al., 2024; Fekete, et al., 2015). | ||||||||||||
| Hyphenated Technique | Principle | Sensitivity | Detection Limit | Precision | Sample Preparation Impact | Advantage | Disadvantage | Best Applications | Resolution | Throughput | Instrument Cost And Maintenance | Suitable Sample Types |
| GC-MS | Combines gas chromatography (separation) with mass spectrometry (detection). | High | Picomolar (extremely low concentrations) | High | High. Often requires derivatization for polar/non-volatile compounds. |
-High chromatographic resolution. -extensive spectral libraries |
-Requires derivatization for non-volatile compounds. -Limited to thermally stable analytes |
Analysis of essential oils, terpenes, and volatile organic compounds | High | High | Moderate | Volatile and semi-volatile compounds |
| HPLC-MS | Combines liquid chromatography (separation) with mass spectrometry (detection) | High | Nanomolar (very low concentrations) | High | Moderate. Requires extraction, filtration, and sometimes derivatization. |
-Broad applicability suitable for thermally labile/non-volatile compounds | -Lower chromatographic resolution than GC -Matrix effects may influence ionization |
Alkaloids, flavonoids, and polar compounds in plant extracts | Moderate | Moderate | High | Polar and non-volatile compounds |
| LC-FTIR | Combines liquid chromatography (separation) with Fourier transform infrared spectroscopy (detection). | Moderate | Nanomolar | Moderate | Moderate. Requires solvent compatibility with FTIR detection. |
-Provides structural information -Non-destructive analysis |
-Lower sensitivity -water interference in IR spectra -Requires specialized detectors |
-Identification of functional groups in complex mixtures | Moderate | Low | High | Samples with functional groups amenable to IR detection |
| LC-NMR-MS | Combines liquid chromatography (separation) with NMR & MS (detection). | Moderate | Micromolar (low concentrations) | High | High. Requires high sample purity, deuterated solvents for NMR, and concentration optimization for sensitivity. The challenge to maintain compatibility with the three techniques while maintaining sample integrity and achieving good signal strength is high. |
-\Comprehensive structural elucidation -Combines molecular weight and structural information |
-High cost -Requires large sample amounts -Complex data interpretation |
-Structure determination of unknown natural products -Metabolite identification |
High | Low | Very High | Samples available in sufficient quantities and complex mixtures requiring detailed analysis |
| UHPLC-MS | Uses ultra-high-pressure liquid chromatography for faster separations with MS. | Very High | Nanomolar | High | Moderate; similar to HPLC-MS but may require finer particle filtration. | Higher resolution and speed than HPLC, reduced solvent consumption, and suitable for high-throughput analysis | -High backpressure -Requires specialized equipment |
-Rapid profiling of natural product extracts -Analysis of complex mixtures with improved resolution |
Very High | Very High | High | Complex mixtures, samples requiring high-throughput analysis |
| LC-UV-MS | Combines liquid chromatography with UV detection and mass spectrometry. | High | Nanomolar | High | Moderate. Requires UV-active compounds for detection. |
Dual detection enhances compound identification -UV provides structural information |
-UV detection limited to chromophoric compounds -Increased complexity in data analysis |
Polyphenols, UV-absorbing compounds, quality control of herbal products | Moderate | Moderate | Moderate | Samples containing UV-active compounds |
| LC-ION-MOBILITY | Combines liquid chromatography with ion mobility spectrometry. | Very High | Nanomolar to picomolar, depending on the ionization source and the detector used | High | Moderate. Less complex than LC-ION-MOBILITY-MS but still requires optimization. |
- Separates compounds based on size, shape, and charge in addition to mass-to-charge ratio. - Resolves isobaric and isomeric compounds that conventional LC-MS may struggle with. - Provides additional structural information through collision cross-section (CCS) values. - Reduces background noise and enhances signal clarity. |
- Requires specialized instrumentation with an additional ion mobility separation module. - Increased complexity in data interpretation. - Higher cost compared to traditional LC-MS. |
- Separation and characterization of isomeric and structurally similar compounds in plant extracts. - study of small molecules, fat, and other related molecules in living organisms where structural differentiation is crucial. - Detection of trace-level secondary metabolites in complex mixtures. |
Very High | Moderate | Very High | Complex natural product extracts containing isomers, samples with interfering matrix effects that require enhanced separation, metabolite profiling in biological and environmental samples. |
| LC-ION-MOBILITY-MS | Combines liquid chromatography with ion mobility separation and MS. | Very High | NANOMOLAR | High | Moderate to high. Requires optimization for ion mobility separation. | Separation based on size, shape, charge -Resolves isobaric compounds -Provides collision cross-section data |
-Requires specialized instrumentation -Complex data interpretation | -Isomer separation -Complex mixture analysis |
Very High | Moderate | Very High | Complex mixtures with isobaric/isomeric compounds, conformational analysis |
| Table 2: First Glance Summary of Reviewed Literatures Showing Techniques used, Country of Study, and Research Focus. | |||
| Technique | Study | Country / Region | Main Research Focus |
| GC-MS | Satyal [46] | USA / Nepal | Essential oils, volatile profiling |
| HPLC-MS | Smith, et al. [81] | USA | Natural product metabolite profiling |
| UHPLC-MS | Johnson, et al. [140] | USA | High-throughput phytochemical analysis |
| GC-MS | Hu, et al. [47] | China | Plant metabolite characterization |
| LC-FTIR | Zhang, et al. [106] | China | Structural elucidation via IR spectra |
| LC-NMR-MS | Wang, et al. [123] | China | Multi-hyphenated structural analysis |
| UHPLC-MS | Chen, et al. [139] | China | High-resolution profiling |
| LC-IM-MS | Zheng, et al. [183] | China | Ion-mobility-based compound separation |
| GC-MS | Lee, et al. [48] | South Korea | Volatile compounds & functional components |
| HPLC-MS | Lee, et al. [83] | South Korea | Bioactive compound identification |
| LC-FTIR | Kim, et al. [107] | South Korea | IR-based structural confirmation |
| LC-NMR-MS | Kim, et al. [124] | South Korea | Comprehensive metabolite structure profiling |
| GC-MS | Patel, et al. [49] | India | Plant extract chemical composition |
| LC-FTIR | Singh, et al. [108] | India | Functional group analysis |
| LC-NMR-MS | Singh, et al. [125] | India | NMR-based metabolite elucidation |
| GC×GC-MS | Garcia-Valverde, et al. 2020 | Spain | Multidimensional volatile analysis |
| UHPLC-MS | García, et al. [118] | Spain | High-resolution metabolomics |
| HPLC-MS | Jones, et al. [82] | UK | Authentication and metabolomics |
| LC-FTIR | Gonzalez, et al. [23] | Mexico | Structural fingerprinting |
| LC-NMR-MS | Tan, et al. [122] | Singapore | Advanced 3D structure elucidation |
| LC-UV-MS | Saidi, et al. [159] | Morocco | UV-based profiling |
| LC-IM / LC-IM-MS | Venter, et al. 2018 | South Africa | Ion mobility separation and profiling |
| LC-IM-MS | Carnevale Neto, et al. [184] | Brazil | Complex metabolite separation |
Recent advancements have resulted in a rise in the use of triple hyphenated techniques, where multiple analytical methods are combined [1]. This approach enables more detailed analysis by integrating separation methods, such as liquid chromatography (LC) or gas chromatography (GC), with various detection techniques, such as mass spectrometry (MS), nuclear magnetic resonance (NMR), and Fourier-transform infrared spectroscopy (FTIR) [190]. For instance, combining LC-MS-MS or LC-NMR-MS facilitates the identification and quantification of compounds in complex matrices, benefiting pharmaceutical and environmental analyses [191]. Hyphenated techniques are becoming increasingly important in natural products analysis, enabling researchers to isolate and identify compounds from complex mixtures efficiently. LC-MS and LC-FTIR techniques are increasingly used for chemotaxonomic studies, chemical fingerprinting, and quality control of herbal products [192]. This trend is motivated by the need for fast and reliable analysis in natural product research, which often involves complex matrices. In addition, innovative sample preparation techniques, such as solid-phase extraction (SPE) and large volume injection (LVI), are being incorporated into hyphenated systems to improve sensitivity and reduce analysis time [193]. These methods help concentrate analytes and remove interfering substances, thereby enhancing the overall performance of the analytical techniques [138]. In the fields of environmental monitoring and food safety, where accurate contaminant detection is crucial, hyphenated techniques are being used with increasing frequency. Research and application in the field of analyzing trace levels of contaminants in environmental samples and monitoring food products for safety and quality are growing [194].
Future developments in hyphenated chromatography techniques will focus on creating increasingly selective and sensitive processes [195]. Mass spectrometry advances like tandem mass spectrometry (MS/MS) and high-resolution mass spectrometry (HRMS) will improve the capacity to evaluate complex samples with lower detection limits and higher accuracy [196]. Also, hyphenated technique automation and instrument miniaturization are trending toward greater accessibility and user-friendliness. This shift is anticipated to speed up sample processing for a variety of applications, including drug development and clinical diagnostics, by enabling high-throughput analysis [197]. Combining cutting-edge technologies like ion mobility spectrometry (IMS) with conventional hyphenated procedures may enhance the study of intricate biological and environmental materials by adding new dimensions of separation and identification [198]. It is anticipated that this hybrid technique would improve analysis speed and resolution. Applications for hyphenated techniques will likely increase in a number of fields, such as metabolomics, forensic science, and biological research, as they continue to advance [199]. It will be easier to find novel compounds and their biological functions and to promote multidisciplinary research efforts if comprehensive analytical data is available [200].
The role of green chromatography approaches in reducing environmental impact
Traditional chromatography methods usually involve the use of large volumes of organic solvents, generate significant laboratory waste, and consume large amounts of energy. To address these challenges, green chromatography approaches are employed [201]. The aim of green chromatography and green chemistry approaches is to enhance biodiversity through eco-friendly sample collection/preparation, enhance safety for laboratory personnel by minimizing exposure to harmful chemicals/substances, reduce solvent dependency, and minimize environmental impact while maintaining analytical performance [202]. Below are a few strategies in green chromatography for hyphenated techniques:
-Use of eco-friendly Solvents [203]
Traditional solvents like acetonitrile and methanol, which are widely used in liquid chromatography, pose environmental and health hazards. To cut down on waste and toxicity, green alternatives such as ethanol, water, and supercritical CO₂ are being explored. CO₂ is used as the mobile phase in supercritical fluid chromatography (SFC), a potent green technology that greatly reduces the use of organic solvents and is a great substitute for natural product analysis.
-Cutting Down on the Use of Solvents and Reagents [203]
Ultra-high-performance liquid chromatography (UHPLC-MS) operates at higher pressures, allowing for shorter columns, quicker run time, and lower solvent consumption while preserving excellent separation efficiency.
-Energy-Efficient Instrumentation
Reduced energy consumption in hyphenated chromatography systems has been facilitated by the development of more effective mass spectrometry (MS) detectors and ionization techniques [204]. Compact and portable chromatography-MS equipment reduces power needs, making field analysis of natural products more feasible [205].
-Recyclable and Biodegradable Materials
Compared to conventional bonded phases, the creation of biodegradable stationary phases, such as silica-based or polymeric columns, reduces the environmental impact. Reusable and recyclable parts for chromatography instruments help minimize laboratory waste [206].
-Solvent Recycling and Waste Management
Solvent recycling methods can recover and purify solvents for reuse in chromatography, thereby significantly reducing waste and operation cost [207].
Advanced waste treatment technologies, such as enzymatic degradation and adsorption-based removal, are currently being integrated into analytical processes to efficiently handle hazardous waste [208].
-Greener Methods for Preparing Samples
Traditional sample preparation methods often require toxic solvents and produce hazardous waste [209]. Green alternatives include Solid-phase micro extraction (SPME) and stir-bar sorptive extraction (SBSE), which use minimal solvents [210]. Also, microwave-assisted and ultrasound-assisted extraction, which enhance extraction efficiency with reduced solvent and energy consumption [211-213].
Novel contributions and advancement of knowledge
This review advances the field of natural product analysis by providing a holistic synthesis of modern hyphenated chromatographic techniques, including LC-NMR-MS, LC-FTIR, GC×GC-MS, UHPLC-MS, and LC-IM-MS, framed not as isolated tools but as an interconnected analytical platform. It emphasizes the complementary qualities of these approaches and their combined capability for handling challenging analytical problems by going beyond single-technique assessments.
This work’s multidisciplinary approach, which compares the usefulness, effectiveness, and limitations of various methods across natural product isolation, environmental monitoring, food safety, and pharmaceutical quality control, is one of its unique features. Making method selection criteria clearer gives researchers a more sophisticated knowledge of which tools are most appropriate for particular applications.
The review also presents an organized framework for green chromatography in hyphenated systems that systematically connects biodegradable stationary phases, energy-efficient detection techniques, eco-friendly solvents, and solvent-recycling methods. It closes a crucial gap between scientific rigor and environmental responsibility by incorporating sustainability into high-performance analytical operations.
This work identifies important directions for future research and technological development by highlighting persistent issues, such as the absence of standardized multi-hyphenated workflows, inadequate spectral libraries for multidimensional datasets, constraints in miniaturization and field deployment, and the conflict between green practices and high-sensitivity detection.
Lastly, the review presents itself as a forward-looking manual that charts the development of hyphenated chromatography toward integrated, high-throughput, and sustainable analytical platforms by examining new trends like IMS-MS, HRMS-enabled workflows, automation, and machine-learning-assisted peak analysis.
Chromatography hyphenated techniques significantly accelerate natural product analysis by providing detailed information about chemical constituents and properties. The future potential of natural product analysis lies in the synergistic combination of various methodologies. This integration will enable scientists to unravel the complex chemical networks found in nature, leading to groundbreaking discoveries in industries such as environmental protection, agriculture, and medicine. GC-MS, HPLC-MS, UHPLC-MS, LC-FTIR, LC-NMR-MS, LC-UV-MS, LC-ION-MOBILITY, and LC-ION MOBILITY each bring unique benefits to the table, from high-resolution separation to detailed molecular characterization. Simultaneously, the shift toward green chromatography ensures that these technological advancements are in line with environmental sustainability goals. Green methods promote safer, more effective, and environmentally friendly analytical procedures by lowering solvent use, energy consumption, and laboratory waste. Together, these trends position hyphenated chromatography as a cornerstone of future analytical research and innovation.
- Awuchi CG, Twinomuhwezi H, Awuchi CG. Hyphenated techniques. In: Analytical Techniques in Biosciences. Academic Press; 2022;125-145. Available from: https://doi.org/10.1016/B978-0-12-822654-4.00015-4
- Ma X. Recent advances in mass spectrometry-based structural elucidation techniques. Molecules. 2022;27(19):6466. Available from: https://doi.org/10.3390/molecules27196466
- Lahlou M. The Success of Natural Products in Drug Discovery. Pharmacology & Pharmacy. 2013;4:17-31. Available from: http://dx.doi.org/10.4236/pp.2013.43A003
- Palazzotto E, Weber T. Omics and multi-omics approaches to study the biosynthesis of secondary metabolites in microorganisms. Current Opinion in Microbiology. 2018;45. Available from: https://doi.org/10.1016/j.mib.2018.03.004
- Piccolella S, Crescente G, Candela L, Pacifico S. Nutraceutical polyphenols: New analytical challenges and opportunities. Journal of Pharmaceutical and Biomedical Analysis. 2019;175:112774. Available from: https://doi.org/10.1016/j.jpba.2019.07.022
- Wixom RL. The beginnings of chromatography—The pioneers (1900–1960). Journal of Chromatography Library. 2001;64:1-38. Available from: https://doi.org/10.1016/S0301-4770(01)80007-5
- Grayson M. A history of gas chromatography mass spectrometry (GC/MS). In: Encyclopedia of Spectroscopy and Spectrometry. Elsevier; 2016;1-10. Available from: https://doi.org/10.1016/B978-0-08-043848-1.00020-1
- Raposo F, Ibelli-Bianco C. Performance parameters for analytical method validation: Controversies and discrepancies among numerous guidelines. TrAC Trends in Analytical Chemistry. 2020;129:115913. Available from: https://doi.org/10.1016/j.trac.2020.115913
- Nagajyothi S, Swetha Y, Neeharika J, Suresh PV, Ramarao N. Techniques comprehensive review. International Journal for Advanced Research and Development. 2017;2(4). Available from: (no URL provided)
- Usman M, Badgujar S, Shaikh T. Hyphenated Techniques of Drug Analysis. Scholars Academic Journal of Pharmacy. 2017;6:263-272. Available from: https://www.researchgate.net/publication/318452941_Hyphenated_Techniques_of_Drug_Analysis
- Verma N. Advances and trends in analytical techniques in natural product research: Challenges and future perspective. Indian Journal of Natural Products and Resources. 2022;12(4):506-526. Available from: https://pdfs.semanticscholar.org/334e/0e8af174d1ea491e5363e7e41f4e1a46ae18.pdf
- Wolfender JL, Marti G, Thomas A, Bertrand S. Current approaches and challenges for the metabolite profiling of complex natural extracts. Journal of Chromatography A. 2015;1382:136-164. Available from: https://doi.org/10.1016/j.chroma.2014.10.091
- Zhang M, Zhao J, Dai X, Li X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations. 2023;10:598. Available from: https://doi.org/10.3390/separations10120598
- Gaudêncio SP, Bayram E, Lukić Bilela L, Cueto M, Díaz-Marrero AR, Haznedaroglu BZ, et al. Advanced methods for natural products discovery: bioactivity screening, dereplication, metabolomics profiling, genomic sequencing, databases and informatics tools, and structure elucidation. Marine Drugs. 2023;21(5):308. Available from: https://doi.org/10.3390/md21050308
- Kumar S, Jaiswal AK, Aggarwal M, Ekbbal R. A Review on Quality Control Aspects of Indian Medicinal Plants. Pharmacognosy Reviews. 2023;17(34). Available from: https://phcogrev.com/sites/default/files/PharmacognRev-17-34-276.pdf
- Sharma B, Yadav DK. Chromatography and hyphenated techniques in quality-based standardization of medicinal plants: Current scenario and future perspectives. South African Journal of Botany. 2023;157:467-483. Available from: https://doi.org/10.1016/j.sajb.2023.04.005
- Kislik V. Chapter 14. Recent Advances in Solvent Extraction Processes and Techniques. 2012. Available from: https://doi.org/10.1016/B978-0-444-53778-2.10014-7
- Lesellier E, Mith D, Dubrulle I. Method development approaches in supercritical fluid chromatography applied to the analysis of cosmetics. Journal of Chromatography A. 2015;1423. Available from: https://doi.org/10.1016/j.chroma.2015.10.053
- Singh R, Mittal S, Sagar NA, Tarafdar A, Sirohi R, Pareek S, Agrawal RK, Kumar K, Pandey A. Chemical analysis of food materials. In: Current Developments in Biotechnology and Bioengineering. Elsevier; 2022;91-118.
- Matei V, Comanescu I, Borcea A. Stationary Phases. 2012. Available from: https://doi.org/10.1007/BF00467689
- Azizi Z, Rezaeimanesh M, Abolghasemi H, Bahmanyar H. Effective diffusivity in a structured packed column: Experimental and Sherwood number correlating study. Chemical Engineering Research and Design. 2014;92:43-53. Available from: https://doi.org/10.1016/j.cherd.2013.07.008
- Sangawitayakorn C, Wilairat P, Chantiwas R. Experimental determination of phase ratio of C8 columns employing retention factors and octane-mobile phase partition coefficients of homologous series of linear alkylbenzenes. Journal of Chromatography A. 2020;1634:461668. Available from: https://doi.org/10.1016/j.chroma.2020.461668
- Gonzalez R, Martinez A, Chavez M. Detection of adulterants in herbal supplements using LC-FTIR. Food Control. 2023;147:109699. Available from: (no URL provided)
- Hareland CA, Guillemot G, Gandin CA, Voorhees PW. The thermodynamics of non-equilibrium interfaces during phase transformations in concentrated multicomponent alloys. Acta Materialia. 2022;241:118407. Available from: https://doi.org/10.1016/j.actamat.2022.118407
- Rusli H, Putri RM, Alni A. Recent developments of liquid chromatography stationary phases for compound separation: from proteins to small organic compounds. Molecules. 2022;27(3):907. Available from: https://doi.org/10.3390/molecules27030907
- Russo M, Camillo MRT, La Tella R, Rigano F, Donato P, Mondello L, Dugo P. Principles and applications of porous graphitic carbon stationary phase in liquid chromatography: an update. Journal of Chromatography A. 2024;464728. Available from: https://doi.org/10.1016/j.chroma.2024.464728
- Ismail BP. Basic Principles of Chromatography. In: Nielsen's Food Analysis. Springer International Publishing; 2024;167-192. Available from: https://doi.org/10.1007/978-3-031-50643-7_12
- Poouthree K, Soonthorntantikul W, Leepipatpiboon N, Petsom A, Nhujak T. Comparison of resolution in microemulsion EKC and MEKC employing suppressed electroosmosis: Application to bisphenol-A-diglycidyl ether and its derivatives. Electrophoresis. 2007;28(20):3705-3711. Available from: https://doi.org/10.1002/elps.200700113
- Lee JW, Row KH. Determination of retention factors of aromatic compounds by gradient-elution reverse-phase high-performance liquid chromatography. Korean Journal of Chemical Engineering. 2002;19(6):978-985. Available from: https://doi.org/10.1007/BF02707220
- Younes OM, Ali FA, Assaf ZA. Enantioseparation of metoprolol tartrate using HPLC by adding methyl β-cyclodextrin to the mobile phase (as chiral additive). Research Journal of Pharmacy and Technology. 2018;11(9):3937-3942. Available from: https://doi.org/10.5958/0974-360X.2018.00723.0
- Misra BB. Advances in high-resolution GC-MS technology: a focus on the application of GC-Orbitrap-MS in metabolomics and exposomics for FAIR practices. Analytical Methods. 2021;13(20):2265-2282. Available from: https://pubs.rsc.org/en/content/articlelanding/2021/ay/d1ay00173f
- Tadikonda RR, Harshitha S, Lahari KL. Gas chromatography-mass spectroscopy: an overview. European Journal of Pharmaceutical Sciences. 2023;10(5):83-89. Available from: https://www.researchgate.net/publication/371989508_GAS_CHROMATOGRAPHY-MASS_SPECTROSCOPY_AN_OVERVIEW
- Hameed IH, Najm FMS, Weiss AHA, Kamel MS. Gas chromatography mass spectrometry (GC-MS): A tool for the health care, environmental, and pharmaceutical industries. Current Clinical and Medical Education. 2024;2(02):43-48. Available from: https://www.visionpublisher.info/index.php/ccme/article/view/50
- Xu S, Yang Y, Su J, Tian Y, Duan Y, Xu Z, Liu Z. The serial coupling-column strategy. Separation & Purification Reviews. 2025;54(3):199-219. Available from: https://doi.org/10.1080/15422119.2024.2379868
- Gruber B, David F, Sandra P. Capillary gas chromatography-mass spectrometry: Current trends and perspectives. TrAC Trends in Analytical Chemistry. 2019;124. Available from: https://doi.org/10.1016/j.trac.2019.04.007
- Ong R, Marriott P, Morrison P, Haglund P. Influence of chromatographic conditions on separation in comprehensive gas chromatography. Journal of Chromatography A. 2002;962:135-152. Available from: https://doi.org/10.1016/s0021-9673(02)00458-2
- Aguilar Meza IB, Ortiz Ortega E, Hosseinian H, Rodríguez Vera A, Rosales López MJ, Hosseini S. Characterization techniques for chromatography analysis. In: Material Characterization Techniques and Applications. Springer Singapore; 2022;221-267. Available from: https://doi.org/10.1007/978-981-16-9569-8_8
- Dümichen E, Eisentraut P, Celina M, Braun U. Automated thermal extraction-desorption gas chromatography mass spectrometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. Journal of Chromatography A. 2019;1592. Available from: https://doi.org/10.1016/j.chroma.2019.01.033
- Bragg L, Qin Z, Alaee M, Pawliszyn J. Field sampling with a polydimethylsiloxane thin-film. Journal of Chromatographic Science. 2006;44:317-323. Available from: https://doi.org/10.1093/chromsci/44.6.317
- Haddad PR, Taraji M, Szücs R. Prediction of analyte retention time in liquid chromatography. Analytical Chemistry. 2020;93(1):228-256. Available from: https://doi.org/10.1021/acs.analchem.0c04190
- Wang H, Li J. Microporous metal–organic frameworks for adsorptive separation of C5–C6 alkane isomers. Accounts of Chemical Research. 2019;52(7):1968-1978. Available from: https://doi.org/10.1021/acs.accounts.8b00658
- Beale DJ, Pinu FR, Kouremenos KA, Poojary MM, Narayana VK, Boughton BA, et al. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics. 2018;14:1-31. Available from: https://doi.org/10.1007/s11306-018-1449-2
- Amaral MS, Nolvachai Y, Marriott PJ. Multidimensional gas chromatography platforms for the analysis of flavours and odorants. In: Comprehensive Analytical Chemistry. Vol. 96. Elsevier; 2022;119-153. Available from: https://doi.org/10.1016/bs.coac.2021.10.005
- Martínez-Pérez-Cejuela H, Gionfriddo E. Evolution of green sample preparation: fostering a sustainable tomorrow in analytical sciences. Analytical Chemistry. 2024;96(20):7840-7863. Available from: https://doi.org/10.1021/acs.analchem.4c01328
- Zhang Y, Wang Y. Recent trends of machine learning applied to multi-source data of medicinal plants. Journal of Pharmaceutical Analysis. 2023;13(12):1388-1407. Available from: https://doi.org/10.1016/j.jpha.2023.07.012
- Satyal P. Development of a GC-MS database of essential oil components by the analysis of natural essential oils and synthetic compounds, and discovery of biologically active novel chemotypes in essential oils. 2015. Available from: https://louis.uah.edu/uah-dissertations/63/
- Hu J, Qi Q, Zhu Y, Wen C, Olatunji OJ, Jayeoye TJ, Eze FN. Unveiling the anticancer, antimicrobial, and antioxidative properties, and UPLC-ESI-QTOF-MS/GC–MS metabolite profile of the lipophilic extract of siam weed (Chromolaena odorata). Arabian Journal of Chemistry. 2023;16(7):104834. Available from: https://doi.org/10.1016/j.arabjc.2023.104834
- Lee EM, Park SJ, Lee JE, Lee BM, Shin BK, Kang DJ, Lee DY. Highly geographical specificity of metabolomic traits among Korean domestic soybeans (Glycine max). Food Research International. 2019;120:12-18. Available from: https://doi.org/10.1016/j.foodres.2019.02.021
- Patel MK. Analysis of volatile organic compounds in breath as a potential diagnostic modality in disease monitoring. 2011. (No URL provided)
- García-Valverde MT, de Medina VS, Codesido V, Hidalgo-García J, Ferreiro-Vera C. Exploring the mysteries of cannabis through gas chromatography. Recent Advances in Gas Chromatography. 2020;65. Available from: https://www.intechopen.com/chapters/74361
- He P, Aga DS. Comparison of GC-MS/MS and LC-MS/MS for the analysis of hormones and pesticides in surface waters: advantages and pitfalls. Analytical Methods. 2019;11(11):1436-1448. Available from: https://doi.org/10.1039/C8AY02774A
- Yang X, Sima Y, Luo X, Li Y, He M. Analysis of GC × GC fingerprints from medicinal materials using a novel contour detection algorithm: a case of Curcuma wenyujin. Journal of Pharmaceutical Analysis. 2024;14(4):100936. Available from: https://doi.org/10.1016/j.jpha.2024.01.004
- Kranenburg RF, García-Cicourel AR, Kukurin C, Janssen HG, Schoenmakers PJ, et al. Distinguishing drug isomers in the forensic laboratory: GC–VUV in addition to GC–MS for orthogonal selectivity and the use of library match scores as a new source of information. Forensic Science International. 2019;302:109900. Available from: https://doi.org/10.1016/j.forsciint.2019.109900
- Samakradhamrongthai RS. The extraction and isolation. In: Aroma and Flavor in Product Development: Characterization, Perception, and Application. Cham: Springer Nature Switzerland; 2024;107-138. Available from: https://www.pharmaexcipients.com/news/aroma-flavor-product-development-2/
- Ranjan S, Roy C, Sinha SK. Gas chromatography–mass spectrometry (GC-MS): a comprehensive review of synergistic combinations and their applications in the past two decades. Journal of Analytical Sciences and Applied Biotechnology. 2023;5(2):72-85. Available from: https://doi.org/10.48402/IMIST.PRSM/jasab-v5i2.40209
- David V, Moldoveanu SC, Galaon T. Derivatization procedures and their analytical performances for HPLC determination in bioanalysis. Biomedical Chromatography. 2021;35(1):e5008. Available from: https://doi.org/10.1002/bmc.5008
- Zaikin VG, Borisov RS. Options of the main derivatization approaches for analytical ESI and MALDI mass spectrometry. Critical Reviews in Analytical Chemistry. 2022;52(6):1287-1342. Available from: https://doi.org/10.1080/10408347.2021.1873100
- Ljoncheva M, Heath E, Heath D, Džeroski S, Kosjek T. Contaminants of emerging concern: silylation procedures, evaluation of the stability of silyl derivatives and associated measurement uncertainty. Science of the Total Environment. 2023;899:165669. Available from: https://doi.org/10.1016/j.scitotenv.2023.165669
- Sadgrove NJ, Padilla-González GF, Phumthum M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants. 2022;11(6):789. Available from: https://doi.org/10.3390/plants11060789
- Misra BB, Olivier M. High-resolution GC-Orbitrap-MS metabolomics using both electron ionization and chemical ionization for analysis of human plasma. Journal of Proteome Research. 2020;19(7):2717-2731. Available from: https://doi.org/10.1021/acs.jproteome.9b00774
- Dutt M, Arigò A, Famiglini G, Palma P, Cappiello A. Chemical ionization mass spectrometry: fundamental principles, diverse applications, and the latest technological frontiers. Mass Spectrometry Reviews. 2025. Available from: https://doi.org/10.1002/mas.70007
- Gritti F, Wahab MF. Understanding the science behind packing high-efficiency columns and capillaries: facts, fundamentals, challenges, and future directions. 2018. Available from: https://www.chromatographyonline.com/view/understanding-science-behind-packing-high-efficiency-columns-and-capillaries-facts-fundamentals-chal
- Dunkle MN, Winniford WL. Petroleum analysis through conventional analytical techniques. In: Analytical Techniques in the Oil and Gas Industry for Environmental Monitoring. 2020;121-160. Available from: https://doi.org/10.1002/9781119523314.ch3
- Siddique IM. Unveiling the power of high-performance liquid chromatography: techniques, applications, and innovations. European Journal of Advances in Engineering and Technology. 2021;8(9):79-84. Available from: https://www.researchgate.net/publication/381305105_Unveiling_the_Power_of_High-Performance_Liquid_Chromatography_Techniques_Applications_and_Innovations
- Klink F. Liquid Chromatography/Mass Spectrometry. 2010. Available from: https://doi.org/10.1002/9780470027318.a6013.pub2
- Kaufmann A. The use of UHPLC, IMS, and HRMS in multiresidue analytical methods: a critical review. Journal of Chromatography B. 2020;1158:122369. Available from: https://doi.org/10.1016/j.jchromb.2020.122369
- Chromnet. Tunneled frit nano-UPLC-MS column. 2024. Available from: http://www.chromnet.net/TunneledFritNano-UPLC-MSColumn.aspx
- Perez de Souza L, Alseekh S, Scossa F, Fernie AR. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nature Methods. 2021;18(7):733-746. Available from: https://doi.org/10.1038/s41592-021-01116-4
- Parys W, Dołowy M, Pyka-Pająk A. Significance of chromatographic techniques in pharmaceutical analysis. Processes. 2022;10(1):172. Available from: https://doi.org/10.3390/pr10010172
- Ganzera M, Sturm S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. Journal of Pharmaceutical and Biomedical Analysis. 2017;147. Available from: https://doi.org/10.1016/j.jpba.2017.07.038
- Shaaban H, Gorecki T. Current trends in green liquid chromatography for the analysis of pharmaceutically active compounds in the environmental water compartments. Talanta. 2015;132:739-752. Available from: https://doi.org/10.1016/j.talanta.2014.09.050
- Claux O, Santerre C, Abert-Vian M, Touboul D, Vallet N, Chemat F. Alternative and sustainable solvents for green analytical chemistry. Current Opinion in Green and Sustainable Chemistry. 2021;31:100510. Available from: https://doi.org/10.1016/j.cogsc.2021.100510
- Chen TL, Kim H, Pan SY, Tseng PC, Lin YP, Chiang PC. Implementation of green chemistry principles in the circular economy system towards sustainable development goals: challenges and perspectives. Science of the Total Environment. 2020;716:136998. Available from: https://doi.org/10.1016/j.scitotenv.2020.136998
- Guzman NA, Guzman DE. A two-dimensional affinity capture and separation mini-platform for the isolation, enrichment, and quantification of biomarkers and their potential use for liquid biopsy. Biomedicines. 2020;8(8):255. Available from: https://doi.org/10.3390/biomedicines8080255
- Thakur A, Tan Z, Kameyama T, El-Khateeb E, Nagpal S, Malone S, et al. Bioanalytical strategies in drug discovery and development. Drug Metabolism Reviews. 2021;53(3):434-458. Available from: https://doi.org/10.1080/03602532.2021.1959606
- Xie T, Kang A, Xu J, Shen C, Zhao X, Di L, Wang S, Shan J. Development of a multiple reaction monitoring (MRM) method based on high-performance liquid chromatography/tandem mass spectrometry to analyze in vivo exposure profiles of complex herbal components independent of standards. RSC Advances. 2016;6. Available from: https://pubs.rsc.org/en/content/articlelanding/2016/ra/c5ra25389f
- Vishwakarma S. LC-MS (Liquid Chromatography–Mass spectrometry). 2021. Available from: https://doi.org/10.13140/RG.2.2.33132.08321
- Donno D, Mellano MG, Gamba G, Riondato I, Beccaro GL. Analytical strategies for fingerprinting of antioxidants, nutritional substances, and bioactive compounds in foodstuffs based on high-performance liquid chromatography–mass spectrometry: an overview. Foods. 2020;9(12):1734. Available from: https://doi.org/10.3390/foods9121734
- Câmara JS, Martins C, Pereira JA, Perestrelo R, Rocha SM. Chromatographic-based platforms as new avenues for scientific progress and sustainability. Molecules. 2022;27(16):5267. Available from: https://doi.org/10.3390/molecules27165267
- Qin Y, Li S, Zhao J. HPLC and HPLC–MS for qualitative and quantitative analysis of Chinese medicines. In: Quality Control of Chinese Medicines: Strategies and Methods. Singapore: Springer Nature Singapore; 2024;475-577. Available from: https://doi.org/10.1007/978-981-99-9871-5_15
- Smith J, Doe A, Brown L. Identification and quantification of bioactive compounds in Panax ginseng using UHPLC-QTOF-MS. Journal of Natural Products. 2022;85(3):123-135.
- Jones M, White S, Green P. Comprehensive metabolomics profiling of the marine sponge Aplysina aerophoba using HPLC-Orbitrap-MS. Marine Drugs. 2023;21(1):45-59.
- Lee K, Kim H, Park J. Quantitative analysis of phytochemicals in dietary supplements using HPLC-MS/MS. Food Chemistry. 2023;371:131-142.
- Assumi SR, Kasomva K, Bhadrecha P. Metabolomics of anticancer plants. In: Plant-Derived Anticancer Drugs in the OMICS Era. Apple Academic Press; 2023;171-213. Available from: https://doi.org/10.1201/9781003377412-8?urlappend=%3Futm_source%3Dresearchgate.net%26utm_medium%3Darticle
- Dhull P, Dunuweera S, Bietsch J, Bandu R, Wannere C, Achanta S, et al. Recent advances and applications of liquid chromatography in the pharmaceutical industry. Journal of Liquid Chromatography & Related Technologies. 2025:1-20. Available from: https://doi.org/10.1080/10826076.2024.2448692
- Bhole RP, Jagtap SR, Chadar KB, Zambare YB. Liquid chromatography-mass spectrometry technique review. Research Journal of Pharmacy and Technology. 2020;13(1):505-516. Available from: https://doi.org/10.5958/0974-360X.2020.00097.9
- Di Marco F, Blöchl C, Esser-Skala W, Schäpertöns V, Zhang T, Wuhrer M, et al. Glycoproteomics of a single protein: revealing tens of thousands of myozyme glycoforms by hybrid HPLC-MS approaches. Molecular & Cellular Proteomics. 2023;22(9):100622. Available from: https://www.mcponline.org/article/S1535-9476(23)00133-0/fulltext
- Halko R, Pavelek D, Kaykhaii M. High-performance liquid chromatography–Fourier transform infrared spectroscopy coupling: a comprehensive review. Critical Reviews in Analytical Chemistry. 2024:1-12. Available from: https://doi.org/10.1080/10408347.2024.2391892
- Guerrero-Pérez MO, Patience G. Experimental methods in chemical engineering: Fourier transform infrared spectroscopy—FTIR. Canadian Journal of Chemical Engineering. 2019;98. Available from: https://doi.org/10.1002/cjce.23664
- Kher MN, Shah RN, Gajjar AK, Chhabria MT, Rakholiya K. Analytical characterization of herbal biomolecules using hyphenated techniques. In: Herbal Formulations, Phytochemistry and Pharmacognosy. Elsevier; 2024;241-253. Available from: https://doi.org/10.1016/B978-0-443-15383-9.00026-3
- Beć KB, Grabska J, Huck CW. Biomolecular and bioanalytical applications of infrared spectroscopy: a review. Analytica Chimica Acta. 2020;1133:150-177. Available from: https://doi.org/10.1016/j.aca.2020.04.015
- Somsen GW, Gooijer C, Velthorst NH, Brinkman UT. Coupling of column liquid chromatography and Fourier transform infrared spectrometry. Journal of Chromatography A. 1998;811(1-2):1-34. Available from: https://doi.org/10.1016/S0021-9673(98)00291-X
- Liu C, Zuo Z, Xu F, Wang Y. Authentication of herbal medicines based on modern analytical technology combined with chemometrics approach: a review. Critical Reviews in Analytical Chemistry. 2023;53(7):1393-1418. Available from: https://doi.org/10.1080/10408347.2021.2023460
- Fahelelbom KMS, Saleh A, Al-Tabakha M, Ashames A. Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: a brief review. Reviews in Analytical Chemistry. 2022;41:21-33. Available from: https://www.degruyterbrill.com/document/doi/10.1515/revac-2022-0030/html
- Shenvi RA. Natural product synthesis in the 21st century: beyond the mountain top. ACS Central Science. 2024;10(3):519-528. Available from: https://doi.org/10.1021/acscentsci.3c01518
- Sharma A, Sharma S, Kumar A, Kumar V, Sharma A. Plant secondary metabolites: an introduction to their chemistry and biological significance with physicochemical aspects. In: [No editors listed]. Springer; 2022. Available from: https://doi.org/10.1007/978-981-16-4779-6_1
- Maehly A, Strömberg L. Chemical criminalistics. Springer Science & Business Media; 2012.
- Friesen JA, Rodwell VW. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biology. 2004;5(11):248. doi:10.1186/gb-2004-5-11-248. PMID:15535874; PMCID:PMC545772. Available from: https://doi.org/10.1186/gb-2004-5-11-248
- Baker M, Trevisan J, Bassan P, Bhargava R, Butler H, Dorling K, et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nature Protocols. 2014;9:1771-1791. Available from: https://doi.org/10.1038/nprot.2014.110
- Gallo M, Ferranti P. The evolution of analytical chemistry methods in foodomics. Journal of Chromatography A. 2016;1428:3-15. Available from: https://doi.org/10.1016/j.chroma.2015.09.007
- Coskun O. Separation techniques: chromatography. North Clin Istanb. 2016;3(2):156-160. doi:10.14744/nci.2016.32757. PMID:28058406; PMCID:PMC5206469. Available from: https://doi.org/10.14744/nci.2016.32757
- Amir R, Anjum F, Khan M, Khan MR, Pasha I, Nadeem M. Application of Fourier transform infrared (FTIR) spectroscopy for the identification of wheat varieties. Journal of Food Science and Technology. 2013;50:1-6. Available from: https://doi.org/10.1007/s13197-011-0424-y
- Salerno TMG, Coppolino C, Donato P, Mondello L. The online coupling of liquid chromatography to Fourier transform infrared spectroscopy using a solute-deposition interface: a proof of concept. Analytical and Bioanalytical Chemistry. 2022;414:703-712. Available from: https://doi.org/10.1007/s00216-021-03693-x
- Somsen G, Visser T. Liquid chromatography/infrared spectroscopy. In: [No editors listed]. Wiley; 2006. Available from: https://doi.org/10.1002/9780470027318.a5608
- Mabuza MJ. Antiplasmodial properties of extracts from Pappea capensis Eckl. & Zeyh. (Sapindaceae) [master’s thesis]. Pretoria (South Africa): University of Pretoria; 2022.
- Zhang X, Li Y, Wang Z. Characterization of essential oils from medicinal plants using LC-FTIR. Journal of Essential Oil Research. 2023;35(1):22-34.
- Kim H, Park J, Lee K. Profiling polyphenolic compounds in green tea using LC-FTIR. Journal of Agricultural and Food Chemistry. 2023;71(4):1230-1241.
- Singh P, Kumar R, Sharma N. Characterization of alkaloids in traditional medicinal plants using LC-FTIR. Phytochemistry Letters. 2023;54:11-20.
- Tiernan H, Byrne B, Kazarian S. ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2020;241:118636. Available from: https://doi.org/10.1016/j.saa.2020.118636
- Margariti C. The application of FTIR microspectroscopy in a non-invasive and non-destructive way to the study and conservation of mineralised excavated textiles. Heritage Science. 2019;7:63. Available from: https://doi.org/10.1186/s40494-019-0304-8
- Dembek M, Bocian S. Pure water as a mobile phase in liquid chromatography techniques. Trends in Analytical Chemistry. 2020;123:115793. Available from: https://doi.org/10.1016/j.trac.2019.115793
- Baviskar KP, Jain DV, Pingale SD, Wagh SS, Gangurde SP, Shardul SA, et al. A review of hyphenated techniques in analytical chemistry. Current Analytical Chemistry. 2022;18(9):956-976. Available from: https://doi.org/10.2174/1573411018666220818103236
- Baiz CR, Błasiak B, Bredenbeck J, Cho M, Choi JH, Corcelli SA, et al. Vibrational spectroscopic map, vibrational spectroscopy, and intermolecular interaction. Chemical Reviews. 2020;120(15):7152-7218. Available from: https://doi.org/10.1021/acs.chemrev.9b00813
- Amouzegar Z, Jalal NR, Kamalabadi M, Tarighat MA, Afkhami A, Madrakian T, et al. Spectrometric miniaturized instruments. In: Micro- and Nanotechnology Enabled Applications for Portable Miniaturized Analytical Systems. Elsevier; 2022; 17-40. Available from: https://doi.org/10.1016/B978-0-12-823727-4.00016-X
- Ashebr T, Linert W, Nayathuparambil M, Birhanu T, Frew R. LC–NMR for natural product analysis: a journey from an academic curiosity to a robust analytical tool. Sci. 2021;3:6. Available from: https://doi.org/10.3390/sci3010006
- Stavrianidi A. A classification of liquid chromatography mass spectrometry techniques for evaluation of chemical composition and quality control of traditional medicines. Journal of Chromatography A. 2020;1609:460501. Available from: https://doi.org/10.1016/j.chroma.2019.460501
- Faixo S, Gehin N, Balayssac S, Gilard V, Mazeghrane S, Haddad M, et al. Current trends and advances in analytical techniques for the characterization and quantification of biologically recalcitrant organic species in sludge and wastewater: a review. Analytica Chimica Acta. 2021;1152:338284. Available from: https://doi.org/10.1016/j.aca.2021.338284
- Garcia-Perez I, Posma JM, Serrano-Contreras JI, Boulange CL, Chan Q, Frost G, et al. Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nature Protocols. 2020;1-30. Available from: https://doi.org/10.1038/s41596-020-0343-3
- Gebretsadik T, Linert W, Thomas M, Berhanu T, Frew R. LC–NMR for natural product analysis: a journey from an academic curiosity to a robust analytical tool. Sci. 2021;3(1):6. Available from: https://doi.org/10.3390/sci3010006
- Kumar S. Recent applications of hyphenated liquid chromatography techniques in food forensics. Eurasian Journal of Analytical Chemistry. 2021;16(2).
- Pawar SV, Ho CHJ, Yadav GD, Yadav GV. The impending renaissance in the discovery & development of natural products. Curr Top Med Chem. 2017;17(2):251-267. Available from: https://doi.org/10.2174/1568026616666160530154649
- Tan H, Li W, Chen X. Comprehensive profiling of secondary metabolites in herbal extracts using LC-NMR-MS. Phytochemistry. 2023;200:113110.
- Wang L, Zhang Y, Liu J. Discovery and characterization of antioxidants in fruit extracts using LC-NMR-MS. Food Chem. 2023;401:134007.
- Kim J, Park S, Lee H. Identification of bioactive compounds in marine algae using LC-NMR-MS. J Nat Prod. 2023;86(2):345-356. Available from: https://doi.org/10.1007/978-981-99-7703-1_17
- Singh R, Kumar P, Sharma N. Characterization of phytochemicals in traditional medicinal plants using LC-NMR-MS. J Ethnopharmacol. 2023;295:115365.
- Rasheed S, Fatima M, Rehman K, Kamal S, Hussain I, Akash MSH. Bioanalytical techniques for the prediction of metabolic activity of drug-metabolizing enzymes. In: Biochemistry of Drug Metabolizing Enzymes. Academic Press; 2022; 399-425. Available from: https://doi.org/10.1016/B978-0-323-95120-3.00022-1
- Liu Y, D’Agostino LA, Qu G, Jiang G, Martin JW. High-resolution mass spectrometry (HRMS) methods for nontarget discovery and characterization of poly- and perfluoroalkyl substances (PFASs) in environmental and human samples. TrAC Trends Anal Chem. 2019;121:115420. Available from: https://doi.org/10.1016/j.trac.2019.02.021
- Emwas AH, Szczepski K, Poulson BG, Chandra K, McKay RT, Dhahri M, et al. NMR is a “gold standard” method in drug design and discovery. Molecules. 2020;25(20):4597. Available from: https://doi.org/10.3390/molecules25204597
- Gathungu RM, Kautz R, Kristal BS, Bird SS, Vouros P. The integration of LC-MS and NMR for the analysis of low molecular weight trace analytes in complex matrices. Mass Spectrom Rev. 2020;39(1-2):35-54. Available from: https://doi.org/10.1002/mas.21575
- Ben-Tal Y, Boaler PJ, Dale HJ, Dooley RE, Fohn NA, Gao Y, et al. Mechanistic analysis by NMR spectroscopy: A user’s guide. Prog Nucl Magn Reson Spectrosc. 2022;129:28-106. Available from: https://doi.org/10.1016/j.pnmrs.2022.01.001
- Zhang T, Yu Y, Han S, Cong H, Kang C, Shen Y, et al. Preparation and application of UPLC silica microsphere stationary phase: A review. Adv Colloid Interface Sci. 2023;103070. Available from: https://doi.org/10.1016/j.cis.2023.103070
- Bharatbhai PN, Surati J, Akbari A, Patel S, Shah K, Solanki D. A review on UHPLC instrumentation with advancements. J Adv Pharm Sci. 2023;1(1):33-44. Available from: http://hbrppublication.com/OJS/index.php/JAPS/article/view/3344
- Wiley Magazine. A primer on LC/NMR/MS. 2014. Available from: https://analyticalscience.wiley.com/content/article-do/primer-lc-nmr-ms
- Wang T, Zhang D, Sun D, Gu J. Current status of in vivo bioanalysis of nano drug delivery systems. J Pharm Anal. 2020;10(3):221-232. Available from: https://doi.org/10.1016/j.jpha.2020.05.002
- Butle S, Kalyankar T, Chidrawar R. Review on ultra performance liquid chromatography: A eminent, sensitive, and high throughput analysis over HPLC. 2024. Available from: https://www.researchgate.net/publication/379346963_Review_On_Ultra_Performance_Liquid_Chromatography_A_Eminent_Sensitive_And_High_Throughput_Analysis_Over_HPLC
- Liu T, Li Y, Xu J, Guo Q, Zhang D, Song L, et al. N-Glycosylation and enzymatic activity of the rHuPH20 expressed in Chinese hamster ovary cells. Anal Biochem. 2021;632:114380. Available from: https://doi.org/10.1016/j.ab.2021.114380
- Ashraf SA, Nazir S, Adnan M, Azad ZRAA. UPLC-MS: An emerging novel technology and its application in food safety. In: Analytical Chemistry—Advancement, Perspectives and Applications. 2020. Available from: https://www.intechopen.com/chapters/72391
- Kanu AB. Recent developments in sample preparation techniques combined with high-performance liquid chromatography: A critical review. J Chromatogr A. 2021;1654:462444. Available from: https://doi.org/10.1016/j.chroma.2021.462444
- Chen X, Li Y, Zhang J. Chemical composition analysis of traditional Chinese medicinal plants using UPLC-MS. J Ethnopharmacol. 2023;256:112984.
- Johnson MW, Thompson CD, Lee AH. Secondary metabolites from marine sponges: Exploring chemical diversity with UPLC-MS. Mar Drugs. 2022;20(4):207.
- García E, Pérez M, Santos J. Polyphenolic content of fruit extracts analyzed by UPLC-MS: Health benefits and applications. Food Chem. 2023;392:134234.
- Plumb RS, Isaac G, Rainville PD, Hill J, Gethings LA, Johnson KA, et al. High throughput UHPLC-MS-based lipidomics using vacuum jacketed columns. J Proteome Res. 2021;21(3):691-701. Available from: https://doi.org/10.1021/acs.jproteome.1c00836
- Svejdal RR, Sticker D, Sønderby C, Kutter JP, Rand KD. Thiol-ene microfluidic chip for fast on-chip sample clean-up, separation, and ESI mass spectrometry of peptides and proteins. Anal Chim Acta. 2020;1140:168-177. Available from: https://doi.org/10.1016/j.aca.2020.09.062
- Khaled O, Ryad L, Nagi M, Eissa F. Development and validation of a multiclass method for the determination of veterinary drug residues in honey. Int J Environ Anal Chem. 2024;1-18. Available from: https://doi.org/10.1016/j.foodchem.2016.11.026
- Chawla G, Ranjan C. Principles, instrumentation, and applications of UPLC: A novel technique of liquid chromatography. Open Chem J. 2016;3:1–16. Available from: https://doi.org/10.2174/1874842201603010001
- Cortese M, Gigliobianco MR, Magnoni F, Censi R, Di Martino P. Compensate for or minimize matrix effects? Strategies for overcoming matrix effects in liquid chromatography-mass spectrometry technique: A tutorial review. Molecules. 2020;25(13):3047. Available from: https://doi.org/10.3390/molecules25133047
- Pacini T, Fu W, Gudmundsson S, Chiaravalle E, Brynjólfsson S, Palsson B, et al. Multidimensional analytical approach based on UHPLC-UV-ion mobility-MS for the screening of natural pigments. Anal Chem. 2015;87(1). Available from: https://doi.org/10.1021/ac504707n
- Zhu X, Huo S, Xue C, An B, Qu J. Current LC-MS-based strategies for characterization and quantification of antibody-drug conjugates. J Pharm Anal. 2020;10(3):209–220. Available from: https://doi.org/10.1016/j.jpha.2020.05.008
- Sarkar J, Singh R, Chandel S. Understanding LC/MS‐based metabolomics: A detailed reference for natural product analysis. Proteomics Clin Appl. 2025;19(1):e202400048. Available from: https://doi.org/10.1002/prca.202400048
- Chaudhary P, Gupta M, Tawar MG, Shrivastava B. Need & scope of standardization of herbal medicines review. J Res Pharm Sci. 2021;7(8):26–31. Available from: http://dx.doi.org/10.2139/ssrn.4961133
- Zhu Y, Chen G, Zhang K, Chen C, Chen W, Zhu M, et al. High-throughput metabolic soft-spot identification in liver microsomes by LC/UV/MS: Application of a single variable incubation time approach. Molecules. 2022;27(22):8058. Available from: https://doi.org/10.3390/molecules27228058
- Mir-Cerdà A, Granados M, Saurina J, Sentellas S. Olive tree leaves as a great source of phenolic compounds: Comprehensive profiling of NaDES extracts. Food Chem. 2024;456:140042. Available from: https://doi.org/10.1016/j.foodchem.2024.140042
- Li N, Simon JE, Wu Q. Determination of anthocyanins, organic acids, and phenolic acids in hibiscus market products using LC/UV/MS. J Food Sci. 2024;89(2):1098–1113. Available from: https://doi.org/10.1111/1750-3841.16909
- Hemida M, Haddad PR, Lam SC, Coates LJ, Riley F, Diaz A, et al. Small footprint liquid chromatography-mass spectrometry for pharmaceutical reaction monitoring and automated process analysis. J Chromatogr A. 2021;1656:462545. Available from: https://doi.org/10.1016/j.chroma.2021.462545
- Son A, Kim W, Park J, Park Y, Lee W, Lee S, et al. Mass spectrometry advancements and applications for biomarker discovery, diagnostic innovations, and personalized medicine. Int J Mol Sci. 2024;25(18):9880. Available from: https://doi.org/10.3390/ijms25189880
- Hemida M, Ghiasvand A, Macka M, Gupta V, Haddad PR, Paull B. Recent advances in miniaturization of portable liquid chromatography with emphasis on detection. J Sep Sci. 2023;46(15):2300283. Available from: https://doi.org/10.1002/jssc.202300283
- Cetinkaya A, Yayla S, Hurkul MM, Ozkan SA. Comprehensive review on chromatographic analysis of flavonoids in fruits. J Chromatogr Open. 2025;7:100209. Available from: https://doi.org/10.1016/j.jcoa.2025.100209
- Lyu W, Yuan B, Liu S, Simon JE, Wu Q. Assessment of lemon juice adulteration by targeted screening using LC-UV-MS and untargeted screening using UHPLC-QTOF/MS with machine learning. Food Chem. 2022;373:131424. Available from: https://doi.org/10.1016/j.foodchem.2021.131424
- Saidi S, Remok F, Handaq N, Drioiche A, Gourich AA, Menyiy NE, et al. Phytochemical profile, antioxidant, antimicrobial, and antidiabetic activities of Ajuga iva (L.). Life. 2023;13(5):1165. Available from: https://doi.org/10.3390/life13051165
- Tinnirello V, Zizzo MG, Conigliaro A, Tabone M, Ganji N, R, Cicio A, et al. Industrial-produced lemon nanovesicles ameliorate experimental colitis-associated damages in rats via the activation of anti-inflammatory and antioxidant responses and microbiota modification. Biomed Pharmacother. 2024;174:116514. Available from: https://doi.org/10.1016/j.biopha.2024.116514
- Alikord M, Mohammadi A, Kamankesh M, Shariatifar N. Food safety and quality assessment: comprehensive review and recent trends in the applications of ion mobility spectrometry (IMS). Critical Reviews in Food Science and Nutrition. 2022;62(18):4833-4866. Available from: https://doi.org/10.1080/10408398.2021.1879003
- Kartowikromo KY, Pizzo JS, Rutz T, Love ZE, Simmons AM, Ojeda A, et al. Identification and Structural Elucidation of Acylsugars in Tomato Leaves Using Liquid Chromatography–Ion Mobility–Tandem Mass Spectrometry (LC-IM-MS/MS). Journal of the American Society for Mass Spectrometry. 2024;36(1):135-145. Available from: https://doi.org/10.1021/jasms.4c00376
- Lee J, Davidson K, Bush M, Kim H. Collision cross sections and ion structures: Development of a general calculation method via high-quality ion mobility measurements and theoretical modeling. The Analyst. 2017;142:5137-5146. Available from: https://doi.org/10.1039/C7AN01276D
- Abdulhussain N, Nawada S, Schoenmakers P. Latest trends on the future of three-dimensional separations in chromatography. Chemical Reviews. 2021;121(19):12016-12034. Available from: https://doi.org/10.1021/acs.chemrev.0c01244
- Popov RS, Ivanchina NV, Dmitrenok PS. Application of MS-based metabolomic approaches in analysis of starfish and sea cucumber bioactive compounds. Marine Drugs. 2022;20(5):320. Available from: https://doi.org/10.3390/md20050320
- Liu L, Wang Z, Zhang Q, Mei Y, Li L, Liu H, et al. Ion mobility mass spectrometry for the separation and characterization of small molecules. Analytical Chemistry. 2023;95(1):134-151. Available from: https://doi.org/10.1021/acs.analchem.2c02866
- Zappi A, Marassi V, Giordani S, Kassouf N, Roda B, Zattoni A, et al. Extracting information and enhancing the quality of separation data: a review on chemometrics-assisted analysis of volatile, soluble, and colloidal samples. Chemosensors. 2023;11(1):45. Available from: https://doi.org/10.3390/chemosensors11010045
- Wu Q, Wang JY, Han DQ, Yao ZP. Recent advances in differentiation of isomers by ion mobility mass spectrometry. TrAC Trends in Analytical Chemistry. 2020;124:115801. Available from: https://doi.org/10.1016/j.trac.2019.115801
- Huang Z, Bi T, Jiang H, Liu H. Review on NMR as a tool to analyse natural products extract directly: Molecular structure elucidation and biological activity analysis. Phytochemical Analysis. 2024;35(1):5-16. Available from: https://doi.org/10.1002/pca.3292
- Kaufmann A, Butcher P, Maden K, Walker S, Widmer M. Does the ion mobility resolving power as provided by commercially available ion mobility quadrupole time-of-flight mass spectrometry instruments permit the unambiguous identification of small molecules in complex matrices? Analytica Chimica Acta. 2020;1107:113-126. Available from: https://doi.org/10.1016/j.aca.2020.02.032
- Mavroudakis L, Lanekoff I. Identification and imaging of prostaglandin isomers utilizing MS3 product ions and silver cationization. Journal of the American Society for Mass Spectrometry. 2023;34(10):2341-2349. Available from: https://doi.org/10.1021/jasms.3c00233
- Song XC, Canellas E, Dreolin N, Goshawk J, Lv M, Qu G, et al. Application of ion mobility spectrometry and the derived collision cross-section in the analysis of environmental organic micropollutants. Environmental Science & Technology. 2023;57(51):21485-21502. Available from: https://doi.org/10.1021/acs.est.3c03686
- Koomen DC, May JC, McLean JA. Insights and prospects for ion mobility-mass spectrometry in clinical chemistry. Expert Review of Proteomics. 2022;19(1):17-31. Available from: https://doi.org/10.1080/14789450.2022.2026218
- Morris CB, Poland JC, May JC, McLean JA. Fundamentals of ion mobility-mass spectrometry for the analysis of biomolecules. Ion Mobility-Mass Spectrometry: Methods and Protocols. 2020:1-31. Available from: https://doi.org/10.1007/978-1-0716-0030-6_1
- Olajide O, Kartowikromo K, Hamid A. Ion mobility mass spectrometry: Instrumentation and applications. IntechOpen. 2023. Available from: https://doi.org/10.5772/intechopen.1002767
- Ross DH, Bilbao A, Smith RD, Zheng X. Ion Mobility Spectrometry-Mass Spectrometry for High-Throughput Analysis. High-Throughput Mass Spectrometry in Drug Discovery. 2023:183-213. Available from: https://doi.org/10.1002/9781119678496.ch6
- Capitain C, Weller P. Non-targeted screening approaches for profiling of volatile organic compounds based on gas chromatography-ion mobility spectroscopy (GC-IMS) and machine learning. Molecules. 2021;26(18):5457. Available from: https://doi.org/10.3390/molecules26185457
- Celma A, Ahrens L, Gago-Ferrero P, Hernández F, López F, Lundqvist J, et al. The relevant role of ion mobility separation in LC-HRMS-based screening strategies for contaminants of emerging concern in the aquatic environment. Chemosphere. 2021;280:130799. Available from: https://doi.org/10.1016/j.chemosphere.2021.130799
- Camunas-Alberca SM, Moran-Garrido M, Sáiz J, Gil-de-la-Fuente A, Barbas C, Gradillas A. Integrating the potential of ion mobility spectrometry-mass spectrometry in the separation and structural characterisation of lipid isomers. Frontiers in Molecular Biosciences. 2023;10:1112521. Available from: https://doi.org/10.3389/fmolb.2023.1112521
- Dodds JN, Hopkins ZR, Knappe DR, Baker ES. Rapid characterization of per- and polyfluoroalkyl substances (PFAS) by ion mobility spectrometry–mass spectrometry (IMS-MS). Analytical Chemistry. 2020;92(6):4427-4435. Available from: https://doi.org/10.1021/acs.analchem.9b05364
- Kirkwood KI, Odenkirk MT, Baker ES. Ion mobility spectrometry. Mass Spectrometry for Lipidomics: Methods and Applications. 2023;1:151-182. Available from: https://doi.org/10.1002/9783527836512.ch6
- Karonen M. Insights into polyphenol–lipid interactions: Chemical methods, molecular aspects, and their effects on membrane structures. Plants. 2022;11(14):1809. Available from: https://doi.org/10.3390/plants11141809
- Zheng W, Li G, Yang G, Lu P, Li Q, Zhang M, et al. Two-dimensional liquid chromatography and ion mobility-mass spectrometry for the multicomponent characterization of different parts of the medicinal plant Gynostemma longipes. Frontiers in Chemistry. 2023;11. Available from: https://doi.org/10.3389/fchem.2023.1203418
- Carnevale Neto F, Clark TN, Lopes NP, Linington RG. Evaluation of ion mobility spectrometry for improving constitutional assignment in natural product mixtures. Journal of Natural Products. 2022;85(3):519-529. Available from: https://doi.org/10.1021/acs.jnatprod.1c01048
- Sonawane D, Sahu AK, Jadav T, Tekade RK, Sengupta P. Innovation in strategies for sensitivity improvement of chromatography and mass spectrometry-based analytical techniques. Critical Reviews in Analytical Chemistry. 2023;53(3):655-671. Available from: https://doi.org/10.1080/10408347.2021.1969887
- Chapman J, Truong VK, Elbourne A, Gangadoo S, Cheeseman S, Rajapaksha P, et al. Combining chemometrics and sensors: Toward new applications in monitoring and environmental analysis. Chemical Reviews. 2020;120(13):6048-6069. Available from: https://doi.org/10.1021/acs.chemrev.9b00616
- Kumar S, Bogusz MJ. Hyphenated techniques in liquid chromatography and their applications in forensic toxicology: A review. Journal of Forensic Science and Medicine. 2021;7(4):123-136. Available from: https://journals.lww.com/jfsm/fulltext/2021/07040/hyphenated_techniques_in_liquid_chromatography_and.3.aspx
- Queiroz EF, Guillarme D, Wolfender J-L. Advanced high-resolution chromatographic strategies for efficient isolation of natural products from complex biological matrices: From metabolite profiling to pure chemical entities. Phytochemistry Reviews. 2024;23:1415-1442. Available from: https://doi.org/10.1007/s11101-024-09928-w
- Fekete S, Veuthey J-L, Guillarme D. Comparison of the most recent chromatographic approaches applied for fast and high-resolution separations: Theory and practice. Journal of Chromatography A. 2015;1408. Available from: https://doi.org/10.1016/j.chroma.2015.07.014
- Letertre MP, Dervilly G, Giraudeau P. Combined nuclear magnetic resonance spectroscopy and mass spectrometry approaches for metabolomics. Analytical Chemistry. 2020;93(1):500-518. Available from: https://doi.org/10.1021/acs.analchem.0c04371
- Halder M, Kundu A, Jha S. Secondary Metabolites Identification Techniques of the Current Era. In: Plant Specialized Metabolites: Phytochemistry, Ecology and Biotechnology. 2024:1-41. Available from: https://doi.org/10.1016/B978-0-443-21818-7.00005-8
- Salunke M, Wakure B, Wakte P. Hyphenated techniques for the characterization of seaweed bioactive compounds. Research Journal of Pharmacy and Technology. 2023;16(9):4455-4461. Available from: https://doi.org/10.52711/0974-360X.2023.00727
- Dugheri S, Mucci N, Bonari A, Marrubini G, Cappelli G, Ubiali D, et al. Liquid phase microextraction techniques combined with chromatography analysis: a review. Acta Chromatographica. 2020;32(2):69-79. Available from: https://doi.org/10.1556/1326.2019.00636
- Fedorenko D, Bartkevics V. Recent applications of nano-liquid chromatography in food safety and environmental monitoring: A review. Critical Reviews in Analytical Chemistry. 2021;53:1-25. Available from: https://doi.org/10.1080/10408347.2021.1938968
- Vishnu KN, Aiswarya KN, Sumesh K. Recent advances in sample preparation in chromatographic techniques. Advances in Separation Sciences. 2025:87-105. Available from: https://doi.org/10.1016/B978-0-323-95292-7.00016-5
- Lacalle-Bergeron L, Izquierdo-Sandoval D, Sancho JV, López FJ, Hernández F, Portolés T. Chromatography hyphenated to high resolution mass spectrometry in untargeted metabolomics for investigation of food (bio)markers. TrAC Trends in Analytical Chemistry. 2021;135:116161. Available from: https://doi.org/10.1016/j.trac.2020.116161
- Elpa DP, Prabhu GRD, Wu SP, Tay KS, Urban PL. Automation of mass spectrometric detection of analytes and related workflows: A review. Talanta. 2020;208:120304. Available from: https://doi.org/10.1016/j.talanta.2019.120304
- Pillai MS, Paritala ST, Shah RP, Sharma N, Sengupta P. Cutting-edge strategies and critical advancements in the characterization and quantification of metabolites concerning translational metabolomics. Drug Metabolism Reviews. 2022;54(4):401-426. Available from: https://doi.org/10.1080/03602532.2022.2125987
- Girel S, Meister I, Glauser G, Rudaz S. Hyphenation of microflow chromatography with electrospray ionization mass spectrometry for bioanalytical applications focusing on low molecular weight compounds: A tutorial review. Mass Spectrometry Reviews. 2024. Available from: https://doi.org/10.1002/mas.21898
- Dzobo K. The role of natural products as sources of therapeutic agents for innovative drug discovery. Comprehensive Pharmacology. 2022:408. Available from: https://doi.org/10.1016/B978-0-12-820472-6.00041-4
- Fuente-Ballesteros A, Ares AM, Bernal J. Paving the way towards green contaminant analysis: Strategies and considerations for sustainable analytical chemistry. Green Analytical Chemistry. 2025;12:100221. Available from: https://ui.adsabs.harvard.edu/abs/2025GAnCh..1200221F/abstract
- Korany M, Mahgoub H, Haggag R, Ragab M, Elmallah O. Green chemistry: Analytical and chromatography. Journal of Liquid Chromatography & Related Technologies. 2017;40. Available from: https://doi.org/10.1080/10826076.2017.1373672?urlappend=%3Futm_source%3Dresearchgate.net%26utm_medium%3Darticle
- De Godoi Passerine BF, Breitkreitz MC. Recent Developments in Green Chromatography. Brazilian Journal of Analytical Chemistry. 2023;10(40):17-34. Available from: https://brjac.com.br/artigos/brjac-126-2022.pdf
- Panda M, Sandhya T, Sameera Bhanu M, Vasudha D, Varaprasadrao K. Advances within the hyphenation of flow analysis techniques. World Journal of Pharmaceutical Research. 2021;10:676-685. Available from: https://www.wisdomlib.org/science/journal/world-journal-of-pharmaceutical-research/d/doc1379751.html
- De Oliveira AM, Vizioli BDC, Castiblanco JEB, de Aguiar Porto N, Hantao LW. Green aspects of multidimensional separation techniques. In: Green Approaches for Chemical Analysis. 2023:173-203. Available from: https://doi.org/10.1016/B978-0-12-822234-8.00001-9
- Goyal S, Sharma R, Singh J, Asadnia M. Green chromatography techniques. In: Green Chemical Analysis and Sample Preparations: Procedures, Instrumentation, Data Metrics, and Sustainability. 2022:379-432. Available from: https://doi.org/10.1007/978-3-030-96534-1_10
- Hessel V, Tran NN, Asrami MR, Tran QD, Long NVD, Escribà-Gelonch M, et al. Sustainability of green solvents–review and perspective. Green Chemistry. 2022;24(2):410-437. Available from: https://doi.org/10.1039/D1GC03662A
- Ahmed SF, Mofijur M, Nuzhat S, Chowdhury AT, Rafa N, Uddin MA, et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. Journal of Hazardous Materials. 2021;416:125912. Available from: https://doi.org/10.1016/j.jhazmat.2021.125912
- Marchel M, Cieśliński H, Boczkaj G. Deep eutectic solvents microbial toxicity: Current state of art and critical evaluation of testing methods. Journal of Hazardous Materials. 2022;425:127963. Available from: https://doi.org/10.1016/j.jhazmat.2021.127963
- Kader MS, Rahman MRT. Supercritical fluid extraction (SFE), solid-phase micro extraction (SPME), and stir bar sorption extraction (SBSE) techniques. Techniques to Measure Food Safety and Quality: Microbial, Chemical, and Sensory. 2021:219-227. Available from: https://doi.org/10.1007/978-3-030-68636-9_10
- Garcia-Vaquero M, Ummat V, Tiwari B, Rajauria G. Exploring ultrasound, microwave, and ultrasound–microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Marine Drugs. 2020;18(3):172. Available from: https://doi.org/10.3390/md18030172
- Gandhi K, Sharma N, Gautam PB, Sharma R, Mann B, Pandey V. Chromatography. In: Advanced Analytical Techniques in Dairy Chemistry. 2022:11-83. Available from: https://doi.org/10.1007/978-1-0716-1940-7_2
- González-López ME, Laureano-Anzaldo CM, Pérez-Fonseca AA, Arellano M, Robledo-Ortíz JR. A critical overview of adsorption models linearization: methodological and statistical inconsistencies. Separation & Purification Reviews. 2022;51(3):358-372. Available from: https://doi.org/10.1080/15422119.2021.1951757