More Information
Submitted: September 22, 2025 | Approved: Ocotober 27, 2025 | Published: Ocotober 28, 2025
How to cite this article: Mansour SR, Moustafa MA, Moustafa MA, Elshazly AM, Moustafa AA. Beyond Conventional Mating: Reviewing the Possibility of Second Pregnancies in Albino Rats without Male Access. Arch Case Rep. 2025; 9(10): 333-338. Available from:
https://dx.doi.org/10.29328/journal.acr.1001169
DOI: 10.29328/journal.acr.1001169
Copyright license: © 2025 Mansour SR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Albino rat; Fertility without male access; Mammalian reproduction; Non-conventional reproduction; Second pregnancy; Pseudopregnancy; Parthenogenesis
Beyond Conventional Mating: Reviewing the Possibility of Second Pregnancies in Albino Rats without Male Access
Samira R Mansour1* , Mostafa A Moustafa2, Mardies A Moustafa3, Amaal M Elshazly1 and Abdelraouf A Moustafa1
, Mostafa A Moustafa2, Mardies A Moustafa3, Amaal M Elshazly1 and Abdelraouf A Moustafa1
1Botany and Microbiology Department, Faculty of Science, Suez Canal University, Ismailia, Egypt
2Neurology Department, Faculty of Medicine, 6 October University, Egypt
3Endodentric Department, Faculty of Dentistry, Saini University, Egypt
*Address for Correspondence: Samira R Mansour, Botany and Microbiology Department, Faculty of Science, Suez Canal University, Ismailia, Egypt, Email: [email protected]
Background: Albino rats are extensively utilized in reproductive and biomedical research owing to their well-characterized physiology. Under normal physiological conditions, a successful pregnancy necessitates copulation with a fertile male. However, atypical cases have been documented wherein females appear to undergo a subsequent pregnancy in the absence of male contact following an initial mating event.
Objective: This review aims to clarify potential biological mechanisms underlying this rare and interesting phenomenon, synthesize evidence from rodent studies and comparative biology, and discuss the implications for reproductive science in the future.
Methods: A narrative review of the literature was performed, focusing on the reproductive physiology of albino rats, pseudopregnancy, sperm storage, superfetation, and parthenogenesis. Additional insights were combined from studies involving other species exhibiting similar reproductive phenomena.
Results: Several mechanisms may explain the occurrence of second pregnancies in the absence of male contact: (a) sperm storage within the female reproductive tract allowing delayed fertilization; (b) hormonally induced pseudopregnancy without fertilization; (c) superfetation, defined as conception during an ongoing pregnancy; (d) accidental parthenogenesis, although this phenomenon is extraordinarily rare in mammals; and (e) experimental or housing artifacts, including overlooked male access. Evidence from rodent models predominantly supports pseudopregnancy and sperm storage as the most reasonable explanations, whereas parthenogenesis remains highly improbable in mammals.
Conclusion: The observation of second pregnancies in albino rats without male presence challenges the traditional paradigm of mammalian reproduction. Although definitive evidence is currently lacking, consideration of these potential mechanisms exposes significant gaps in reproductive biology knowledge. Future studies employing genetic, hormonal, and histological approaches are needed to clarify this phenomenon and its implications for reproductive biology, laboratory animal management, and the reliability of experimental outcomes.
The phenomenon of pregnancy in the absence of male participation has long fascinated reproductive biologists, challenging traditional understandings of fertilization and pregnancy. True parthenogenesis can be simply defined as a form of asexual reproduction where an egg develops into an embryo without fertilization. This phenomenon has been documented in various species, predominantly in invertebrates and some reptiles [1,2]. Recent studies have confirmed facultative and obligate parthenogenesis in diverse taxa, including sharks [3], lizards and snakes [2], and even in some amphibians [4]. These findings reveal that parthenogenesis serves as an adaptive reproductive strategy in species facing environmental or demographic challenges, facilitating reproduction in the absence of males while contributing to their evolutionary flexibility and biodiversity. A confirmed case of true parthenogenesis in mammals would revolutionize our understanding of genetics, embryonic development, and evolutionary biology.
Albino rats serve as one of the most widely utilized model organisms in reproductive and biomedical research due to their well-understood physiology, ease of breeding, and genetic uniformity. Their reproductive biology has been extensively characterized, demonstrating that successful pregnancy generally requires copulation with a fertile male. This initial understanding supports numerous studies related to fertility, endocrinology, and developmental biology [5,6]. However, recent reports have challenged this conventional view by reporting cases where female albino rats appear to undergo subsequent pregnancies without any male contact [7], raising important questions about the complexities and possible alternative mechanisms in mammalian reproduction [7].
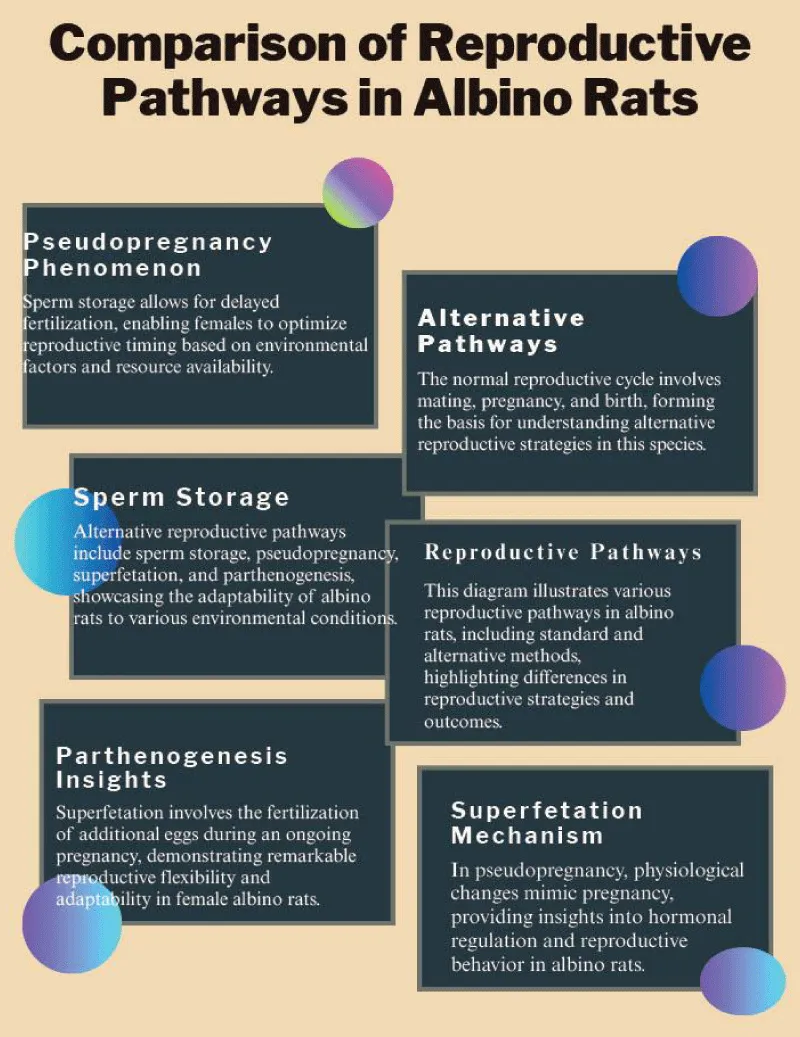
The phenomenon of a second pregnancy also suggests the existence of alternative pathways for reproductive activation, including spontaneous ovulation, pseudo-pregnancy, or overlooked environmental and physiological factors (Figure 1). Such pathways may operate independently of copulation, indicating more complex regulatory mechanisms in female reproductive physiology than previously understood. These findings not only have significant implications for our understanding of reproductive biology but also impact laboratory animal management, breeding programs, and experimental reliability. For instance, unrecognized pregnancies or reproductive states can confound experimental outcomes, leading to variability and challenges in reproducibility. Moreover, these occurrences raise important questions about the triggers and controls of ovulation and implantation, as well as the potential for cryptic fertilization events. Understanding whether these events are due to physiological phenomena, such as sperm storage, hormonal states, or rare reproductive capabilities like parthenogenesis, is crucial to interpreting experimental data accurately. Furthermore, elucidating these mechanisms could inform improvements in animal husbandry protocols, ensuring more precise control over breeding and reducing unintended reproductive events that may affect both scientific studies and animal welfare [8].
Figure 1: Comparison of reproductive pathways in albino rats, highlighting standard and alternative mechanisms including pseudopregnancy, sperm storage, superfetation, and parthenogenesis. The figure illustrates the physiological adaptability of albino rats to diverse environmental conditions through multiple reproductive strategies.
This review aims to explain existing knowledge and explore the potential biological mechanisms that could elucidate such extraordinary reproductive events. By examining data from rodent studies and comparative biology across species, the review seeks to clarify whether known reproductive phenomena, such as sperm storage, pseudopregnancy, superfetation, or parthenogenesis, can account for these observations. Further, it highlights the gaps in current understanding and discusses the implications for reproductive science, laboratory practices, and future research directions.
Currently, there is limited or no documented evidence of second pregnancies occurring in albino rats in the absence of subsequent male access. This underscores the rarity or potential absence of natural parthenogenesis or alternative reproductive mechanisms in this species. Conventional mammalian reproductive biology generally presumes that male fertilization is essential for the initiation of subsequent pregnancies. Most studies on albino rat reproduction report normal gestation periods and reproductive cycles that involve male involvement for fertilization. Exceptional reproductive phenomena such as superfecundation are rare and involve complex mechanisms. The present study investigates an underexplored aspect of albino rat reproduction by suggesting the possibility of second pregnancies occurring without male contact. This observation challenges the traditional understanding of mammalian reproduction and warrants further investigation into potential non-conventional reproductive mechanisms in this species.
This hypothetical observation is scientifically extraordinary, as it would challenge the established understanding of obligate sexual reproduction in placental mammals. The main items concerning this study would focus on rigorously investigating the purported event, considering established biological mechanisms that could mimic pregnancy, and exploring more radical, but less likely, biological explanations. Primary hypotheses and probable mechanisms for understanding this rare occurrence could provide new insights into reproductive physiology and potential parthenogenetic or hormonally induced processes.
Explanations for the second pregnancy mechanisms in rats
1. Pseudopregnancy misinterpretation: The most reasonable explanation of the observed “second pregnancy” is actually pseudopregnancy, in which rats, cervical stimulation (from infertile mating, interactions with other females, or environmental factors) activates a neuroendocrine reflex. Environmental factors may play a role in regular fertility, where any disturbance can delay pregnancy [9]. The most important effective factor is light. Light exposure significantly influences reproductive physiology in albino rats through its effects on the hypothalamic-pituitary-gonadal (HPG) axis and the circadian regulation of reproductive hormones. Albino rats, which possess amelanotic retinas, are particularly sensitive to light intensity and photoperiod changes. Exposure to abnormal lighting conditions, such as constant light or light at night, disrupts their reproductive cycles by altering the secretion of key hormones, including luteinizing hormone (LH), follicle-stimulating hormone (FSH), and progesterone, ultimately affecting ovulation and fertility [10-12].
2. Hormonal state: This response prolongs the life of the corpus luteum, which secretes progesterone. Elevated progesterone causes physiological changes, mammary gland development, nesting behavior, and weight gain that mimic true pregnancy [13,14]. An investigative procedure can be done through hormonal profiling (progesterone, prolactin) during suspected gestation to distinguish true pregnancy from pseudopregnancy.
3. Delayed implantation: A possible explanation for the occurrence of a second pregnancy in albino rats is an overlooked mating during the fertile postpartum estrus, which typically occurs within approximately 48 hours after parturition. Mating during this brief fertile window can initiate a new pregnancy; however, the implantation of embryos may be delayed or enter a dormant state [15]. This delay in implantation allows the uterus to temporarily pause embryonic development until maternal conditions become favorable for embryo attachment and further gestation.
Delayed implantation, also known as embryonic diapause, is a well-documented reproductive strategy observed in numerous mammalian species, allowing synchronization of birth with optimal environmental conditions and maternal physiological readiness [16]. In rodents, including albino rats, this facultative delay is regulated by a balance of ovarian hormones, primarily progesterone and estrogen. Studies demonstrate that the preimplantation surge of ovarian estrogen is critical for blastocyst activation and uterine receptivity, and its absence or insufficiency can maintain the blastocyst in a dormant state [17].
During delayed implantation, the blastocyst exhibits reduced metabolic activity and fails to initiate uterine attachment until it receives appropriate hormonal signals [18,19]. This dormancy can last from several days to weeks, after which the blastocyst is reactivated, leading to resumed growth and implantation. Thus, in albino rats, embryos conceived during postpartum estrus might remain in a state of delayed implantation, explaining the observation of a delayed or second pregnancy.
Lactation delay
The process of lactation and suckling induces a neuroendocrine response that temporarily inhibits uterine receptivity and embryo implantation, resulting in a state of embryo dormancy or diapause. This phenomenon, known as lactational delay, is a form of facultative delayed implantation widely documented in rodents. It involves hormonal regulation primarily through sustained progesterone secretion, which maintains pregnancy, and the suppression of ovarian estrogen surges necessary for blastocyst activation and uterine readiness [20-22]. This adaptive reproductive strategy allows rodents, including albino rats, to optimize reproductive success by spacing pregnancies according to physiological and environmental constraints.
Prolactin secretion, stimulated by suckling, acts centrally on the hypothalamic-pituitary-gonadal (HPG) axis to suppress the preimplantation estrogen surge critical for blastocyst activation and subsequent implantation. This hormonal regulation postpones implantation until maternal conditions, such as weaning and adequate energy reserves, are suitable for supporting pregnancy [23].
Subsequent implantation
When suckling intensity decreases or the first litter is stopped, the inhibitory neuroendocrine signals disappear, permitting blastocysts to transition from a metabolically dormant state to an active state capable of implantation in the uterine lining, thereby initiating a subsequent pregnancy. This process ensures optimal maternal resource allocation and successful establishment of pregnancy under physiologically favorable conditions [24].
Superfetation and or sequential ovulation
Some mammals can perceive a second fetus while already pregnant. This refers to the rare occurrence in which a female considers a second fetus while already pregnant. Although this phenomenon can explain overlapping pregnancies, it necessitates fertilization by sperm and is distinct from delayed implantation. Superfetation requires a permissive hormonal and physiological environment that allows ovulation and fertilization during an ongoing gestation [25].
To investigate these mechanisms, careful monitoring of mating behavior during the postpartum fertile period, controlled litter separation to modulate suckling stimuli, and comparative analysis of gestation length relative to established delays in implantation are essential. Furthermore, hormonal assays measuring progesterone, estrogen, and prolactin, combined with molecular markers of blastocyst activation, can provide robust evidence supporting delayed implantation as the underlying process for observed secondary pregnancies without apparent new mating events [26]. Another potential mechanism involves the retention of viable sperm within the female reproductive tract from prior mating events, allowing fertilization of a new cohort of ova at a later stage. This hypothesis warrants further investigation for anatomical or physiological peculiarities that may enable extended sperm viability [27].
Sperm retention and storage
Sperm viability explores the less-documented possibility of long-term sperm retention in the female’s reproductive tract, considering what research would be needed to test this hypothesis, including precise timing of male isolation relative to copulation.
Long-term sperm storage, the least likely explanation, within the female reproductive tract is not characteristic of rat physiology, where sperm viability typically persists for only several hours to a few days post-copulation. Unlike some vertebrate species, such as certain reptiles and bats, that possess specialized anatomical adaptations enabling extended sperm preservation, rats lack such mechanisms. In the meantime, long-term sperm storage in the female tract is not characteristic of rat physiology, but it remains a theoretical possibility.
Recent advances in assisted reproductive technologies demonstrate improved in vitro sperm storage capabilities; however, these do not reflect natural physiological processes [28]. Nevertheless, the possibility of passing sperm retention or rare exceptions in rodents cannot be entirely dismissed and warrants further investigation [29]. Detailed studies utilizing histological examinations and molecular markers are essential to elucidate whether any cryptic anatomical or physiological phenomena could facilitate prolonged sperm viability in the rat female tract. This line of inquiry may reveal novel reproductive adaptations or anomalies influencing fertility outcomes.
In this study, female albino rats aged 7 to 8 weeks, weighing approximately 190 ± 30 grams, were monitored to investigate the occurrence of second pregnancies without male access. The rats were housed in standard conditions with controlled temperature, humidity, and a 12-hour light/dark cycle. After an initial confirmed pregnancy through vaginal smear examination, the pregnant female rats were observed continuously for behavioral and physiological changes, which indicated a possible second pregnancy during the subsequent postpartum period. Key observations included changes in physical appearance, weight, and reproductive behavior. The timing of pregnancies and inter-pregnancy intervals was carefully recorded. The timing of pregnancies was varied based on the female rat itself, which was recorded between 21 to 40 days after the first pregnancy. This case-based approach allowed us to document and analyze the phenomenon within a controlled experimental framework, linking individual animal cases to broader reproductive biology insights [30].
Future prospective
This phenomenon can open up ideas on different levels:
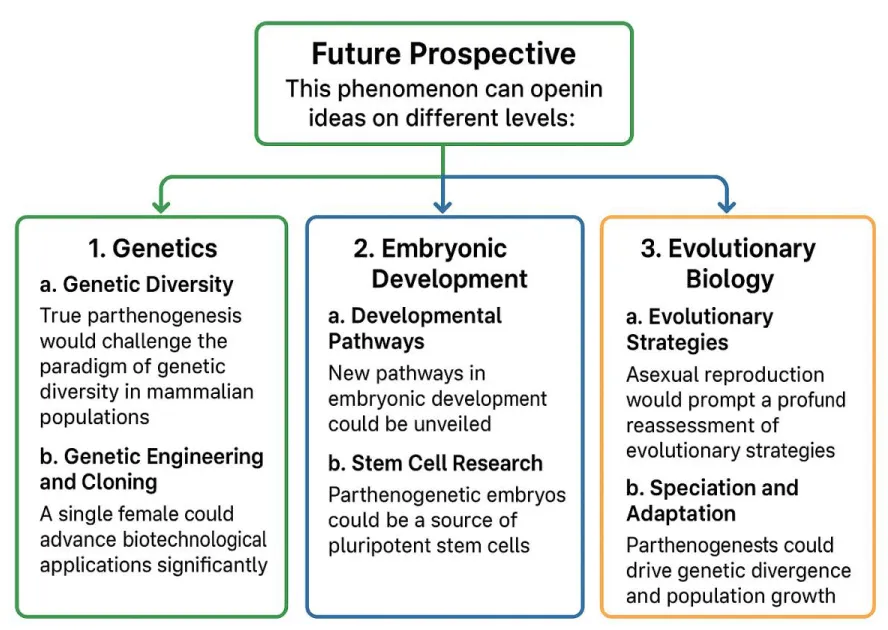
1. Genetics
A. Genetic diversity: Currently, sexual reproduction promotes genetic diversity through the combination of parental DNA. True parthenogenesis would challenge this paradigm, as offspring would be genetically identical to the mother. This could lead to a reevaluation of the importance of genetic diversity in mammalian populations and the mechanisms that drive evolution.
B. Genetic engineering and cloning: The ability of a single female to generate viable offspring has the potential to advance biotechnological applications significantly. This capability could lead to the development of techniques for cloning or producing genetically modified organisms without the requirement for male gametes. Such advancements would streamline research in genetics and developmental biology by simplifying reproduction-related methodologies.
C. Epigenetics: Mammalian embryo development is governed by complex epigenetic regulation involving DNA methylation, histone modifications, and chromatin remodeling. A parthenogenetic embryo offers a unique model to study how epigenetic factors influence development in the absence of paternal genetic contributions. Investigating epigenetic imprinting in such embryos can provide valuable insights that may reshape our understanding of heredity, gene expression regulation, and developmental processes.
2. Embryonic development
A. Developmental pathways: True parthenogenesis could unveil new pathways in mammalian embryonic development. The mechanisms by which an egg can develop independently may reveal alternative developmental routes that are not observed in sexually reproducing mammals.
B. Stem cell research: Parthenogenetic embryos could serve as a source of pluripotent stem cells, offering a unique avenue for regenerative medicine. If these cells retain the ability to differentiate into various cell types, they could be used for therapeutic purposes without ethical concerns related to embryo destruction. Parthenogenetic embryos have garnered significant interest as a valuable source of pluripotent stem cells. These cells possess the capacity to differentiate into a wide range of specialized cell types, making them particularly promising for regenerative medicine applications. Importantly, stem cells derived from parthenogenetic embryos offer unique ethical advantages, as their derivation does not involve the destruction of viable embryos, thereby bypassing many ethical concerns associated with embryonic stem cell research.
3. Evolutionary biology
A. Evolutionary strategies: The occurrence of true parthenogenesis in mammals would prompt a profound reassessment of established evolutionary strategies. Sexual reproduction is traditionally favored due to its ability to generate genetic diversity, which enhances adaptability and resilience in changing environments. However, the emergence of asexual reproductive modes, such as parthenogenesis, challenges this paradigm by allowing for reproduction without males, resulting in offspring genetically identical to the mother. From an evolutionary perspective, parthenogenesis may confer selective advantages under specific ecological conditions, such as low population density, scarcity of mates, or stable environments where rapid population expansion is beneficial. This reproductive strategy enables a lineage to proliferate quickly without the genetic cost or risk associated with sexual reproduction, such as the production of less fit male offspring or the need to find a mate.
Nevertheless, evolutionary trade-offs exist; the lack of genetic recombination in parthenogenetic reproduction can reduce genetic variation, potentially limiting long-term adaptability and increasing vulnerability to environmental changes or diseases. This balance between the benefits of rapid reproduction and the risks of reduced genetic diversity raises critical questions regarding the ecological contexts and evolutionary pressures that could allow mammalian parthenogenesis to evolve and persist. Understanding these dynamics in albino rats or other model organisms can provide insights into the plasticity of reproductive modes and the complex interplay between genetics, environment, and evolutionary fitness.
Recent advances in mammalian biology suggest that although natural parthenogenesis in mammals is generally considered highly unlikely due to genomic imprinting and developmental constraints, experimental evidence demonstrates that parthenogenetic development can be induced under controlled conditions, and in some cases, viable offspring have been produced [31]. This indicates that while rare and constrained, the possibility of parthenogenesis in mammals should not be dismissed entirely and could have important evolutionary and biomedical implications.
B. Speciation and adaptation: Parthenogenesis alters established concepts of speciation by introducing evolutionary pathways distinct from those observed in sexual reproduction. This mode of reproduction can drive genetic divergence by enabling populations to become reproductively isolated in the absence of genetic recombination. By allowing organisms to reproduce independently of males, parthenogenesis facilitates rapid population growth and colonization in specific environmental contexts where sexual reproduction may be less efficient or hindered.
Furthermore, parthenogenetic lineages may adapt to their environments differently, often by maintaining advantageous gene combinations that support niche specialization. While parthenogenesis limits genetic diversity and can constrain adaptive potential over long timeframes, it simultaneously promotes biodiversity by fostering ecological differentiation and speciation through alternative evolutionary trajectories. Recent molecular studies suggest that, despite inherent genetic and epigenetic challenges, facultative or rare parthenogenetic events might contribute to the diversification and adaptation of mammalian species [31] (Figure 2).
Figure 2: Prospects of true parthenogenesis in mammals.
The possibility of true parthenogenesis would be highlighted as a revolutionary claim that would require irrefutable, replicated evidence before being accepted by the scientific community. Any future study must utilize highly experimental controlled conditions to eliminate confounding variables. Using video monitoring to confirm the absence of male rats or other forms of contact, as well as to precisely document all behavioral and physiological changes. The study should include both truly isolated virgin females and known pseudopregnant females as controls for comparison. A multi-pronged approach is necessary to understand the observed phenomenon for send pergenancy with male contact. Histological and hormonal evaluation could be conducted as hormonal assays and examine reproductive tissues of the affected female to confirm pregnancy and rule out pseudopregnancy or other pathologies. High-resolution genetic testing of offspring is critical to conclusively rule out paternal DNA contribution to the mother’s to rule out any paternal contribution. Given the profound implications, the event must be successfully replicated in a controlled setting. The study would involve carefully designed experiments to test each of the proposed mechanisms. If a repeatable, asexually derived pregnancy is observed, it would necessitate a paradigm shift in the field of reproductive biology.
- Moreira MO, Fonseca C, Rojas D. Parthenogenesis is self-destructive for scaled reptiles. Biol Lett. 2021;17(5):20210006. Available from: https://doi.org/10.1098/rsbl.2021.0006 .
- Booth W, Levine BA, Corush JB, Davis MA, Dwyer Q, De Plecker R, Schuett GW. Discovery of facultative parthenogenesis in a New World crocodile. Biol Lett. 2023;19:20230129. Available from: https://doi.org/10.1098/rsbl.2023.0129
- Esposito G, Meletiadis A, Sciuto S, Prearo M, Gagliardi F, Corrias I, et al. First report of recurrent parthenogenesis as an adaptive reproductive strategy in the endangered common smooth-hound shark Mustelus mustelus. Sci Rep. 2024;14:17171. Available from: https://doi.org/10.1038/s41598-024-67804-1
- Davis MG. INHS researchers reveal “virgin birth” in a crocodile [Internet]. 2023. Available from: https://blogs.illinois.edu/view/7447/817183762
- Quesenberry KE, Boschert KR. Breeding and reproduction of rats. MSD Veterinary Manual [Internet]. 2024. Available from: https://www.msdvetmanual.com/all-other-pets/rats/breeding-and-reproduction-of-rats
- Stramek AK, Johnson ML, Taylor VJ. Improved timed-mating, non-invasive method using fewer unproven female rats with pregnancy validation via early body mass increases. Lab Anim. 2019;53(2):148–159. Available from: https://doi.org/10.1177/0023677218774076
- Ochiogu I, Uchendu C, Ihedioha J. A new and simple method of confirmatory detection of mating in albino rats (Rattus norvegicus). Anim Res Int. 2006;3(3):527–530. Available from: https://www.ajol.info/index.php/ari/article/view/40784
- Eccard J, Dammhahn M, Ylönen H. The Bruce effect revisited: is pregnancy termination in female rodents an adaptation to ensure breeding success after male turnover in low densities? 2017;185(1):81-94. Available from: https://doi.org/10.1007/s00442-017-3904-6
- Abd El-Aziz Y, Kazlak M, Farouk N, Helmy M, Mohamed A, Awad N, Hamed R, Abu Almaaty A. Protective effects of Turbo cornutus extract against HgCl₂-induced pathological changes in albino rat tissues. Catrina: Int J Environ Sci. 2024;31(1):87–98. Available from: https://cat.journals.ekb.eg/article_371481_877a6ddd4f29d054c698da933b75733a.pdf
- Lawton KA, Schwartz NB. The effects of constant light on ovulation in the rat. Endocrinology. 1967;80(2):333–341.
- Brown-Grant K, J Davidson JM, Greig F. Induced ovulation in albino rats exposed to constant light. J Reprod Fertil. 1973;35:27–32. Available from: https://joe.bioscientifica.com/view/journals/joe/57/1/joe_57_1_002.xml
- Emmer KM, Russart KLG, Walker WH, Nelson RJ, DeVries AC. Effects of light at night on laboratory animals and research outcomes. Behav Neurosci. 2018;132(4):302–314. Available from: https://doi.org/10.1037/bne0000252
- Vazakidou P, Bouftas N, Heinzelmann M, Johansson HKL, Svingen T, Leonards PEG, van Duursen MBM. Minor changes to circulating steroid hormones in female rats after perinatal exposure to diethylstilbestrol or ketoconazole. Reprod Toxicol. 2024;130:108726. Available from: https://doi.org/10.1016/j.reprotox.2024.108726
- El Hennawi DE, Ahmed M, El Tabbakh M, Abdelrehem A, Dessouki A. The effect of sildenafil citrate (Viagra®) on nasal olfactory mucosa of adult male albino rat: caspase-3 and inducible nitric oxide synthase protein expressions. Catrina: Int J Environ Sci. 2024;29(1):1–8. Available from: https://doi.org/10.21608/cat.2023.238196.1205
- Agrati D, Uriarte N. What can challenging reproductive contexts tell us about the rat’s maternal behavior? Front Behav Neurosci. 2023;17:1239681. Available from: https://doi.org/10.3389/fnbeh.2023.1239681
- Renfree MB, Shaw G. Diapause. Annu Rev Physiol. 2000;62:353–375. Available from: https://doi.org/10.1146/annurev.physiol.62.1.353
- Zhang Y, Hu M, Yang F, Zhang Y, Ma S, Zhang D, Wang X, Sferruzzi-Perri AN, Wu X, Brännström M, Shao LR, Billig H. Increased uterine androgen receptor protein abundance results in implantation and mitochondrial defects in pregnant rats with hyperandrogenism and insulin resistance. J Mol Med (Berl). 2021;99(10):1427–1446. Available from: https://doi.org/10.1007/s00109-021-02104-z
- Alabiad MA, Elhasadi I, Alnasser SM, Alorini M, Alshaikh ABA, Jaber FA, Shalaby AM, Samy W, Heraiz AI, Mohammed Albakoush KM, Khairy DA. Effect of aromatase inhibitor letrozole on the placenta of adult albino rats: a histopathological, immunohistochemical, and biochemical study. Iran J Med Sci. 2024;49(1):46–56. Available from: https://doi.org/10.30476/ijms.2023.96905.2853
- Hiraoka T, Aikawa S, Mashiko D, Nakagawa T, Shirai H, Hirota Y, Kimura H, Ikawa M. An ex vivo uterine system captures implantation, embryogenesis, and trophoblast invasion via maternal-embryonic signaling. Nat Commun. 2025;16(1):5755. Available from: https://doi.org/10.1038/s41467-025-60610-x
- Lee JE, Oh HA, Song H, Jun JH, Roh CR, Xie H, Dey SK, Lim HJ. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011;152(5):2067–2075. Available from: https://doi.org/10.1210/en.2010-1456
- Yomogita H, Miyasaka N, Kanai-Azuma M. A review of delayed delivery models and the analysis of parturition mechanisms in genetically modified mice. Reprod Med Biol. 2022; 20;10(2):20. Available from: https://doi.org/10.3390/jdb10020020
- Gale T, Garratt M, Brooks RC. Perceived threats of infanticide reduce maternal allocation during lactation and lead to elevated oxidative damage in offspring. Functional Ecology. 2018;32(9): 2158-2169. Available from: https://doi.org/10.1111/1365-2435.13146
- UW Medicine. How some mammals pause their pregnancies. 2020. Available from: https://newsroom.uw.edu/news-releases/how-some-mammals-pause-their-pregnancies
- Wang F, Li S, Meng L, Kuang Y, Liu Z, Ma X. Delayed implantation induced by letrozole in mice. bioRxiv [Preprint]. 2022;29(10):2864-2875. Available from: https://doi.org/10.1007/s43032-022-00902-5
- Pinto-Pinho P, Pinto ML, Monteiro J, Fardilha M, Pinto-Leite R, Colaço B. Pregnancy complications and feto-maternal monitoring in rabbits. Vet Sci. 2023;10(10):622. Available from: https://doi.org/10.3390/vetsci10100622
- He B, Zhang H, Wang J, Liu M, Sun Y, Guo C, Lu J, Wang H, Kong S. Blastocyst activation engenders transcriptome reprogramming affecting X-chromosome reactivation and the inflammatory trigger of implantation. Proc Natl Acad Sci U S A. 2019;116(33):16621–16630. Available from: https://doi.org/10.1073/pnas.1900401116
- Ahmadkhani N, Hosseini M, Saadatmand M, Abbaspourrad A. The influence of the female reproductive tract and sperm features on the design of microfluidic sperm-sorting devices. J Assist Reprod Genet. 2022;39(1):19–36. Available from: https://doi.org/10.1007/s10815-021-02377-w
- Yamaga K, Nakao S, Mikoda N, Sztein JM, Nakagata N, Takeo T. High-concentration bovine serum albumin enhances the fertilization rate of cold-stored rat sperm. Reprod Med Biol. 2024;23(1):e12456. Available from: https://doi.org/10.1262/jrd.2023-085
- Miller DJ. Sperm in the mammalian female reproductive tract: surfing through the tract to try to beat the odds. Annu Rev Anim Biosci. 2024;12: 301-319. Available from: https://doi.org/10.1146/annurev-animal-021022-040629
- Schwartz SM. Effects of constant bright illumination on reproductive behavior in female rats. Physiol Behav. 1982; 6(3):391-406. Available from: https://doi.org/10.1016/0149-7634(82)90049-5
- Wei Y, Yang CR, Zhao ZA. Viable offspring derived from single unfertilized mammalian oocytes. Proc Natl Acad Sci U S A. 2022;119(12):e2115248119. Available from: https://doi.org/10.1073/pnas.2115248119