More Information
Submitted: October 15, 2025 | Approved: Ocotober 21, 2025 | Published: Ocotober 22, 2025
How to cite this article: Sener C, Ergen H, Guleli M, Caliskan C. Development and Validation of Stability Stability-indicating HPLC Method for Related Substances Analysis of Fluorometholone in an Ophthalmic Solution. Arch Case Rep. 2025; 9(10): 326-332. Available from:
https://dx.doi.org/10.29328/journal.acr.1001168
DOI: 10.29328/journal.acr.1001168
Copyright license: © 2025 Sener C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Fluorometholone; RP-HPLC; Stress testing; Stability indicating; Related substances; Validation
Development and Validation of Stability Stability-indicating HPLC Method for Related Substances Analysis of Fluorometholone in an Ophthalmic Solution
Cigdem Sener , Harun Ergen
, Harun Ergen , Muge Guleli
, Muge Guleli and Cem Caliskan*
and Cem Caliskan*
World Medicine Pharmaceutical Industry and Trade Inc. R&D Center, 15 Temmuz Mah. Camiyolu Cad. No:50 K:4 Günesli, İstanbul, Turkey
*Address for Correspondence: Cem Caliskan, World Medicine Pharmaceutical Industry and Trade Inc. R&D Center, 15 Temmuz Mah. Camiyolu Cad. No:50 K:4 Günesli, İstanbul, Turkey, Email: [email protected]
Anti-inflammatory ophthalmic solutions containing fluorometholone (FLM) combinations are commonly used, and very few stability-indicating liquid chromatographic methodshave been reported for their related substances. In this study, a simple HPLC method was developed and validated for the determination of fluorometholone impurities in an ophthalmic solution containing FLM and tetrahydrozoline hydrochloride. All impurities were separated by using gradient elution at a flow rate of 1.5 ml/min using a µBondapak C18 250 mm × 4.6 mm, 5 µm (or equivalent) column kept at 40 °C with 20 µl injection volumes. The wavelength was 240 nm. This method validation included specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, and robustness. The stability-indicating capability of this method was evaluated by performing forced degradation stress studies.
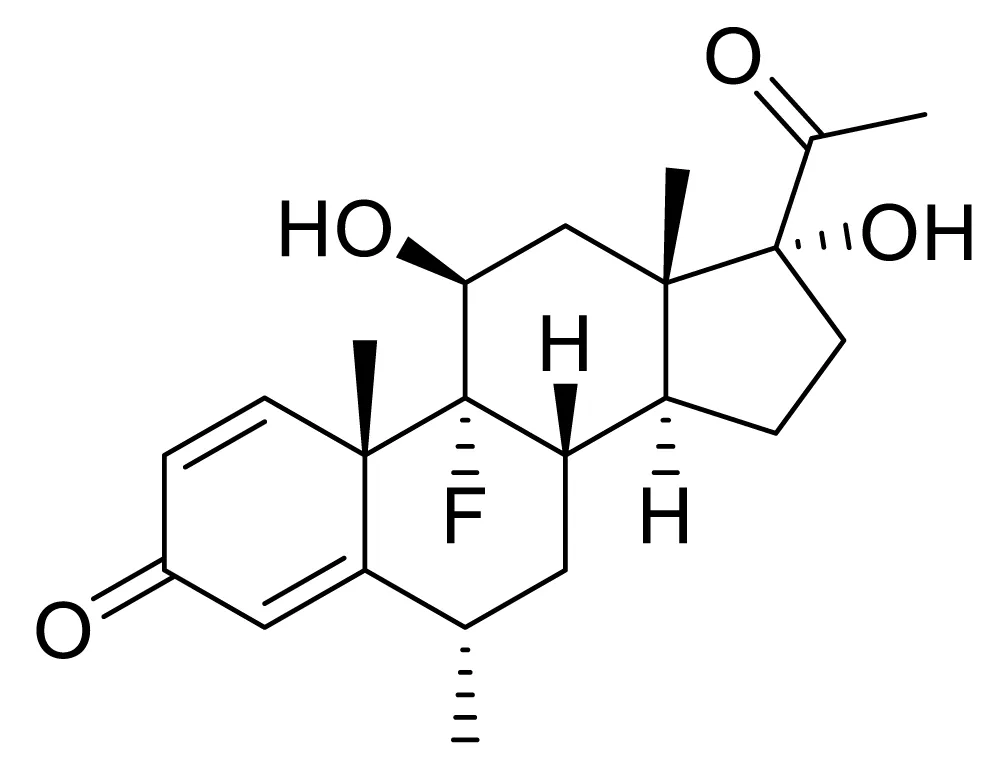
Fluorometholone (FLM), which is a corticosteroid that inhibits inflammation, and its combinations, such as FLM-ketorolac, FLM-tetrahydrozoline hydrochloride, are used in the case of allergic and inflammatory conditions in the eyes, eye redness/itching [1-3] (Figure 1).
Figure 1: Chemical structure of FLM.
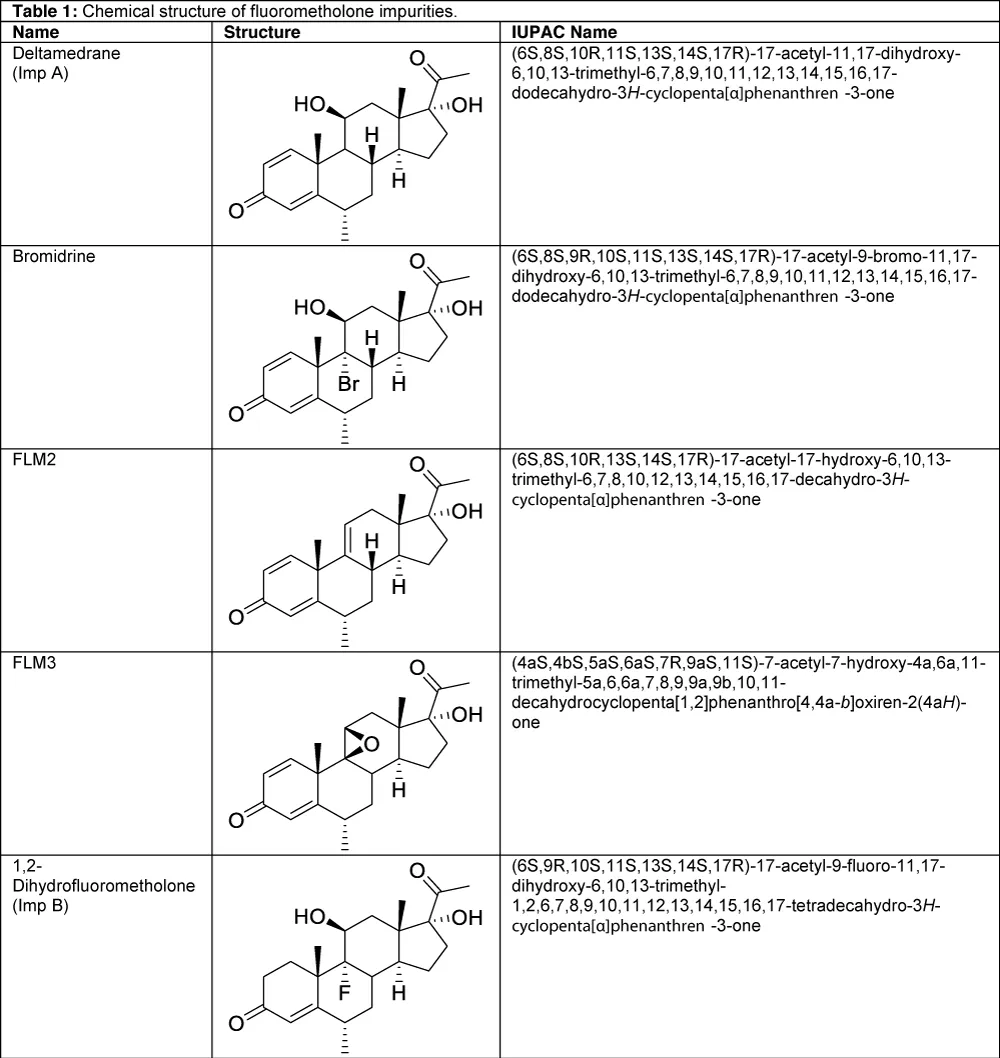
The pharmacopeia survey finds that analytical methods are reported for solutions drops in British (BP) [4], US (USP) Pharmacopeias, and cream, ophthalmic suspension, and ointment only in USP [5]. It is not reported related substances method is used for cream and ointment. Known impurities are not defined for ophthalmic solutions and suspensions in both USP and BP. For the FLM raw material monograph, only the USP identifies one of the known impurities, which is FLM-related compound A (Table 1). In this method, five FLM impurities (Table 1) were successfully separated from each other and detected. Analytical methods for the determination of fluorometholone in drugs were described, including HPTLC for impurity [6], UV spectrophotometry [7], TLC-spectrodensitometry [7], and HPLC for assay [2,9].
Table 1: Chemical structure of fluorometholone impurities.
Materials
Fluorometholone, impurities, and ophthalmic solution containing FLM and tetrahydrozoline hydrochloride sample were taken from commercial batches produced by the World Medicine Pharmaceutical Industry and Trade Inc. (Istanbul, Turkey). Analytical reference standards used during development and validafollows and their purities given in parentheses; fluorometholone (99.32%) and impurities Deltamedrane (95.7%), 1,2-Dihydrofluorometholone (100.0%), Bromidrine (100.0%), FLM2 (96.8%), FLM3 (100.0%). Sodium hydroxide (NaOH), hydrochloric acid (HCl), hydrogen peroxide (H2O2), triethylamine (Et3N), potassium dihydrogen phosphate, and o-phosphoric acid were purchased from Merck, methanol from J. T. Baker, and tetrabutylammonium hydroxide solution (40%) from Acros. HPLC-grade water (0.05 µc) was produced by the Sartorius Stedim Biotech system.
Chromatographic conditions and preparation of solutions
Preparation of mobile phase A:
Mix 500 ml purified water and 500 ml methanol in a 1000 ml volumetric flask and adjust pH to 3.2 ± 0.05 with concentrated phosphoric acid and degase.
Preparation of mobile phase B:
Purified water: Methanol: Phosphoric acid (97: 3: 0.05) (v: v: v).
Related substances HPLC method: The HPLC method developed for related substances analysis of fluorometholone was carried out on µBondapak (250 mm × 4.6 mm, 5 µm) column with 20 µl injection volume at a wavelength of 240 nm on a Waters Alliance E2695 separation module equipped with a Waters 2489 photodiode array (PDA) detector and 2998 UV detector, an Empower-pro data handling system (Waters Corporation, Milford, MA, USA). Column and sample temperatures were 40 ˚C and 25 ˚C, respectively. The separation was employed using gradient elution based on the programsin Table 2. Methanol is used as a dilution solution, and all prepared solutions were filtered through a 0.45 µm PTFE filter.
| Table 2: Program of the method. | ||
| Time (min.) | Mobile Phase A (%) | Mobile Phase B (%) |
| 0 | 60 | 40 |
| 20 | 80 | 20 |
| 50 | 80 | 20 |
| 55 | 90 | 10 |
| 65 | 100 | 0 |
| 75 | 100 | 0 |
| 76 | 60 | 40 |
| 85 | 60 | 40 |
Preparation of standard solution: Weigh accurately about 10.0 mg Fluorometholone into a 100 ml volumetric flask, add some dilution solution and sonicate in an ultrasonic bath until dissolved, complete to volume with dilution solution and mix (Stock solution 1). Transfer 5.0 ml of the obtained solution into a 50 ml volumetric flask, complete to volume with dilution solution, and mix well. Transfer 5.0 ml of the obtained solution into a 50 ml volumetric flask, complete to volume with dilution solution, and mix. Filter through a PTFE filter with a pore size of 0.45 µm and take an HPLC vial.
Preparation of system suitability solution: Weigh accurately 2.0 mg 1,2-Dihydroderivated (Imp B) into a 50 ml volumetric flask, add 30.0 ml stock solution 1, sonicate in an ultrasonic bath until dissolved, complete to volume with stock solution 1, and mix. Transfer 0.5 ml of the obtained solution into a 20 ml volumetric flask, complete to volume with stock solution 1, and mix well. Filter through a PTFE filter with a pore size of 0.45 µm and take an HPLC vial.
Preparation of the sample solution: Take ~1.0 ml sample equivalent to 1.0 mg Fluorometholone into a 10 ml volumetric flask. Add some dilution solution and sonicate in an ultrasonic bath until dissolved, complete to volume with dilution solution, and mix. Filter through a PTFE filter with a pore size of 0.45 µm and take an HPLC vial.
Stress-testing and stability studies: The ophthalmic solution containing FLM and tetrahydrozoline hydrochloride sample were subjected to stress-testing under the following conditions: thermal degradation (standing at 60 °C 14 days), photolytic degradation under day-ligat 25 °C for 14 days, acidic hydrolysis (standing at 5.0 M HCl solution at 25 °C for 14 days and at 60 °C for 14 days), alkaline hydrolysis (standing at 5.0 M NaOH solution at 25 °C for 14 days and at 60 °C for 14 days), and oxidative degradation (standing at 30% H2O2 solution at 25 °C for 14 days). The acidic, basic, and oxidative hydrolysis solutions were neutralized by using 5.0 M NaOH, 5.0 M HCl, and 30% tetrabutylammonium solutions. For each study, corresponding blank solutionanalyzed to determine the formed degradation impurities and.
Optimization of chromatographic conditions
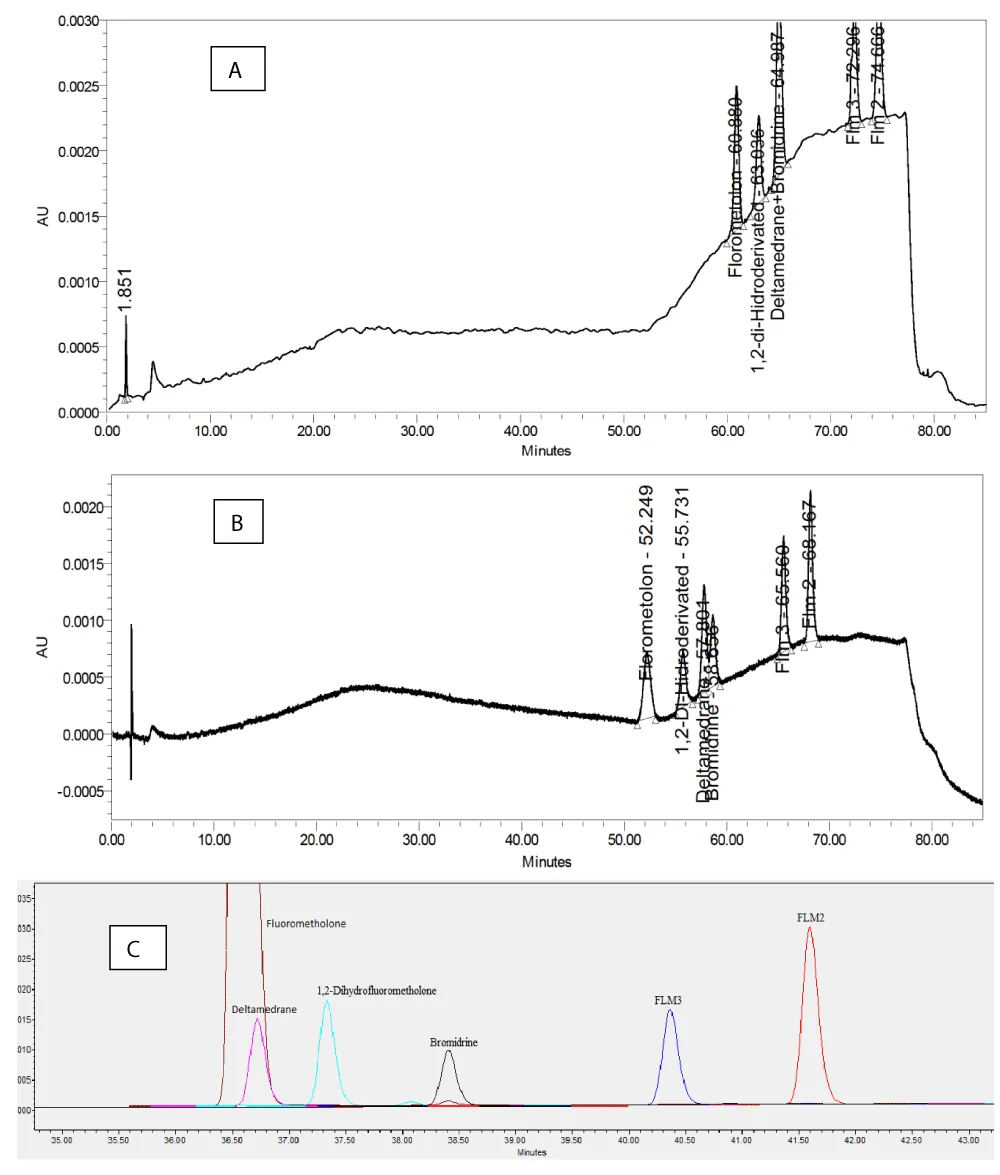
To obtain a good resolution among impurities and FLM, different columns were tested having C18 stationary phases with particle sizes of 10.0 µm diameters of 4.6 mm and 3.9 mm, and column lengths of 250 mm and 300 mm. It was found that impurities and FLM were well retained and separated with µBondapak C18 250 mm × 4.6 mm, 5 µm column. Since the pH level of the mobile phase may affect the chromatographic behaviors, after several trials, an ideal pH was found 3.2 for good resolution. When the pH was not adjusted, the resolution was lost between Deltamedrane impurity and FLM peaks (Figure 2).
Figure 2: Optimization Chromatograms: A; column: ODS Hypersil 250 mm × 4.6 mm, 5 µm and mobile phase: Purified water: Methanol (1:1, v:v) adjusted pH:3.2, B; column: µBondapak C18 250 mm × 4.6 mm, 5 µm and mobile phase: Purified water: Methanol (1:1, v:v) adjusted pH:3.2, C; column: µBondapak C18 250 mm × 4.6 mm, 5 µm and mobile phase: Purified water: Methanol (1:1, v:v).
Ation of HPLC-related substances method
The optimized HPLC method was validated according to the ICH Q2 (R2) guideline [9,10]. The validation parameters included system suitability, specificity, linearity, accuracy, precision (system, method, and intermediate precision), and robustness.
Specificity
In the specother peak was observed on the dilution and placebo solution chromatogram at the retimpurity peaks, which were all separated from each other and found spectrally pure (purity angle < purity threshold) (Suppl. Mat. Table S1). According to the results, the specificity of the developed method was found suitable for the determination of related impurities in FLM/ tetrahydrozoline hydrochloride ophthalmic solution.
System suitability
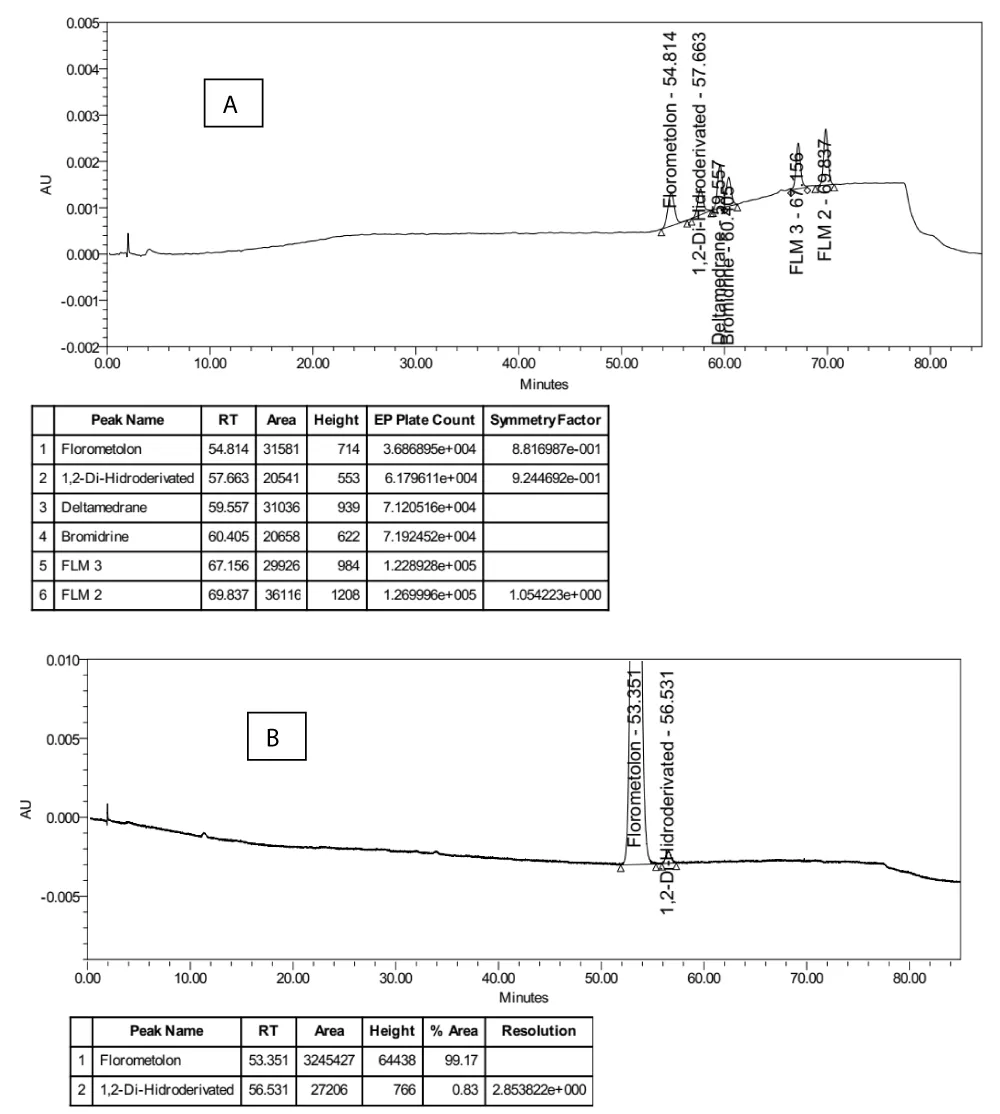
The system was approved to be suitable for use as the resolution between fluorometholone and 1,2-dihydrofluorometholone peaks was found to be 2.9 (≥2.0) in the chromatogram of the system suitability solution; symmetry factor was 0.9 (0.8 - 1.5), and theoretical plate count was 36869 (≥10000) obtained from the standard solution (Figure 3).
Figure 3: HPLC chromatograms: (A) mixture solution of fluorometholone and spiked with impurities at the limit concentration, (B) system suitability solution.
Linearity and range
The linearity of peak areas was checked using different concentrations of standard solution from LOQ to 140%. The calibration curve has shown good linearity with the regression equation y = 31742569.104155x -154.473550 for FLM, and the correlation coefficient (r) was found to be 0.9998. The slope, intercept, and regression coefficient values of impurities were given in Table 3. The linearity graphics obtained from the Empower were given in the Suppl. Mat. S.3.2.3.
| Table 3: Results for linearity and range studies. | |||
| Compound | Concentration (μg/ml) | Correlation coefficient (r) | Regression equation |
| Fluorometholone | 0.050 – 1.402 | 0.9998 | y = 31742569.104155x – 154.473550 |
| Deltamedrane | 0.053 – 1.487 | 0.9998 | y = 29461192.055379x – 8.185844 |
| 1,2-Dihydrofluorometholone | 0.052 – 1.463 | 0.9996 | y = 19580893.497977x + 109.987692 |
| Bromidrine | 0.051 – 1.414 | 0.9995 | y = 20173296.309443x + 25.492796 |
| FLM 3 | 0.050 – 1.407 | 0.9999 | y = 29216002.244713x + 50.449443 |
| FLM 2 | 0.052 – 1.450 | 0.9999 | y = 35137462.922738x – 23.666053 |
Limit of detection (LOD), limit of quantitation (LOQ), and relative response factor (RRF)
The LOD and LOQ were determined at a signal-to-noise ratio of 3:1 and 10:1, respectively. The determined LOD, LOQ, and RRF values for FLM and its impurities were reported in Table 4.
| Table 4: Results for LOD, LOQ, and relative response factor. | ||||||||
| Compound | LOD | LOQ | S/N | RRF | CRF | |||
| µg/ml | % | µg/ml | % | LOD | LOQ | |||
| Fluorometholone | 0.015 | 0.015 | 0.050 | 0.050 | 3.70 | 10.64 | 1.0 | 1.0 |
| Deltamedrane | 0.016 | 0.016 | 0.053 | 0.052 | 3.04 | 10.55 | 0.6 | 1.6 |
| 1,2-Dihydrofluorometholone | 0.016 | 0.016 | 0.052 | 0.053 | 3.01 | 10.05 | 0.9 | 1.1 |
| Bromidrine | 0.015 | 0.015 | 0.051 | 0.051 | 2.96 | 10.05 | 0.6 | 1.6 |
| FLM 3 | 0.015 | 0.015 | 0.050 | 0.050 | 2.86 | 9.32 | 0.9 | 1.1 |
| FLM 2 | 0.016 | 0.016 | 0.052 | 0.052 | 3.00 | 10.83 | 0.9 | 1.1 |
Accuracy
The accuracy was evaluated by measuring recovery through spiking known amounts of the impurities and FLM stinto to placebo containing tetrahydrozoline hydrochloride. Three different concentration levels 80%, 100%, and 120% were prepared and injected three times. Three samples were prepared at each level, and each sample was injected in triplicate. Good-to-excellent recoveries of impurities were achieved within the limit range of 80.0% – 120.0% levels (Table 5).
| Table 5: Results for accuracy. | |||||
| Compound | Accuracy (n = 3) | ||||
| Spiked amount (%) | Conc. (μg/ml) | Conc. found (Mean, μg/ml) |
RSD (%) | Recovery (%) | |
| Deltamedrane | 80 | 0.849 | 0.837 | 0.76 | 98.53 |
| 100 | 1.062 | 1.056 | 0.26 | 99.39 | |
| 120 | 1.274 | 1.275 | 0.66 | 100.01 | |
| 1,2-Dihydrofluorometholone | 80 | 0.836 | 0.839 | 0.60 | 100.34 |
| 100 | 1.045 | 1.032 | 1.44 | 98.77 | |
| 120 | 1.254 | 1.247 | 0.92 | 99.47 | |
| Bromidrine | 80 | 0.808 | 0.811 | 1.63 | 100.37 |
| 100 | 1.010 | 0.987 | 0.82 | 97.72 | |
| 120 | 1.212 | 1.211 | 0.96 | 99.95 | |
| FLM 3 | 80 | 0.804 | 0.808 | 0.65 | 100.56 |
| 100 | 1.005 | 1.015 | 0.67 | 100.98 | |
| 120 | 1.206 | 1.223 | 0.63 | 101.41 | |
| FLM 2 | 80 | 0.829 | 0.819 | 0.41 | 98.82 |
| 100 | 1.036 | 1.035 | 0.49 | 99.91 | |
| 120 | 1.243 | 1.237 | 0.62 | 99.49 | |
Precision and intermediate precision
Thaccount its repeatability and intermediate precision aspects. Repeatability was determined by injecting six individual preparations of a mixture solution of fluorometholone and spiked with impurities at the limit concentration solution in the same equipment on the same day, and the RSD of peak areas was found below 10.0%. To determine the precision mixture solution of Fluorometholone and its impurities at the limit concentration was prepared and injected six times.A sample solution was injected once to determine the amount of impurities. Six sample solutions spiked with impurities at of specification limit concentrations were prepared, and each was injected once. The intermediate precision was also checked by different analysts on different days using different equipment by working like precision. The RSD values of the contents of the detected impurities were calculated Tables 6,7).
| Table 6: Results for precision. | ||||||
| Compound | System precision RSD (%) | Method Precision (n = 6) | ||||
| Conc. (μg/ml) | Conc. found (μg/ml) | Recovery (%) | RSD (%) | Confidence interval at 95% | ||
| Fluorometholone | 0.81 | – | – | – | – | – |
| Deltamedrane | 1.20 | 1.062 | 1.053 | 99.11 | 0.56 | 0.99 – 1.00 |
| 1,2-Dihydrofluorometholone | 1.56 | 1.045 | 1.048 | 100.24 | 0.56 | 1.00 – 1.01 |
| Bromidrine | 1.91 | 1.045 | 1.032 | 98.75 | 0.78 | 0.98 – 1.00 |
| FLM 3 | 0.68 | 1.096 | 1.005 | 101.04 | 0.76 | 1.00 – 1.02 |
| FLM 2 | 0.82 | 1.036 | 1.031 | 99.50 | 0.19 | 0.99 – 1.00 |
| Maximum unknown impurity | – | – | – | – | 2.63 | 0.16 – 0.17 |
| Total impurity | – | – | – | – | 0.43 | – |
| Table 7: Results for intermediate precision. | ||||||
| Compound | Intermediate Precision (n = 6) | RSD (%) (n = 12) | ||||
| Conc. (μg/ml) | Conc. found (μg/ml) | Recovery (%) | RSD (%) | Confidence interval at 95% | ||
| Fluorometholone | – | – | – | – | – | – |
| Deltamedrane | 1.034 | 1.009 | 100.41 | 0.51 | 1.00 – 1.01 | 0.85 |
| 1,2-Dihydrofluorometholone | 1.030 | 1.039 | 100.85 | 0.62 | 1.00 – 1.02 | 0.65 |
| Bromidrine | 1.050 | 1.045 | 99.34 | 1.01 | 0.98 – 1.00 | 0.91 |
| FLM 3 | 1.050 | 1.051 | 100.08 | 0.51 | 1.00 – 1.01 | 0.79 |
| FLM 2 | 1.045 | 1.045 | 100.00 | 0.27 | 1.00 – 1.00 | 0.34 |
| Maximum unknown impurity | – | – | – | 3.03 | 0.15 – 0.16 | 3.22 |
| Total impurity | – | – | – | 0.24 | – | 0.35 |
Robustness
Into investigate the robustness of the method, minor but important changes in method parameters (column temperature by ±2 °C, pH of mobile phase A by ±0.05 units, using a different column) were made, and standard solutions spiked with impurities at the specification limit were tested. % Variation was calculated, and no significant difference was found between initial and altered conditions (Table 8).
| Table 8: Results for robustness. | |||||
| Compound | % Variation for Using Different Column | Column temperature | pH of mobile phase A | ||
| % Variation for 38 °C |
% Variation for 42 °C |
% Variation for pH 3.15 |
% Variation for pH 3.25 |
||
| Fluorometholone | 1.35 | 0.06 | 0.80 | 0.59 | 0.35 |
| 1,2-Dihydrofluorometholone | 0.39 | 0.07 | 1.54 | 0.12 | 0.39 |
| Deltamedrane | 2.38 | 1.09 | 0.77 | 0.53 | 1.88 |
| Bromidrine | 0.26 | 1.38 | 0.60 | 0.12 | 0.80 |
| FLM 3 | 0.69 | 0.16 | 1.45 | 1.13 | 2.26 |
| FLM 2 | 3.57 | 1.19 | 0.84 | 0.13 | 2.57 |
Stabilityyof standard and sample solutions
Solution stability was also evaluated by monitoring the peak area response. Impurity spiked standard and sample solutions were analyzed right aftertheir preparation, 6, 24, and 48 hours after at 5 °C and at room temperature. Standard and sample solutions were stable for 48 hours since the variations were below 10.0% Tables 9,10).
| Table 9: Results for standard solution stability. | ||||||
| Compound | Standard solution at 5 °C | Standard solution at 25 °C | ||||
| % Variation for 6 hours |
% Variation for 24 hours |
% Variation for 48 hours |
% Variation for 6 hours |
% Variation for 24 hours |
% Variation for 48 hours |
|
| Fluorometholone | 2.06 | 2.72 | 1.69 | 0.76 | 0.14 | 1.69 |
| 1,2-Dihydrofluorometholone | 0.00 | 2.63 | 1.34 | 0.15 | 0.43 | 1.01 |
| Deltamedrane | 0.81 | 2.51 | 2.87 | 0.10 | 1.37 | 2.04 |
| Bromidrine | 0.49 | 0.63 | 0.54 | 0.30 | 1.10 | 0.78 |
| FLM 3 | 0.73 | 0.68 | 1.36 | 1.62 | 1.50 | 0.60 |
| FLM 2 | 0.41 | 2.48 | 2.29 | 0.85 | 2.25 | 2.57 |
| Maximum unknown impurity | – | – | – | – | – | – |
| Total impurity | – | – | – | – | – | – |
| Table 10: Results for sample solution stability. | ||||||
| Compound | Sample solution at 5 °C | Sample solution at 25 °C | ||||
| % Variation for 6 hours |
% Variation for 24 hours |
% Variation for 48 hours |
% Variation for 6 hours |
% Variation for 24 hours |
% Variation for 48 hours |
|
| Fluorometholone | 0.91 | 0.93 | 2.04 | 0.14 | 3.37 | 4.48 |
| 1,2-Dihydrofluorometholone | 4.79 | 1.08 | 0.80 | 0.85 | 1.74 | 1.55 |
| Deltamedrane | 1.83 | 1.42 | 1.89 | 2.28 | 0.72 | 2.38 |
| Bromidrine | 7.03 | 1.73 | 0.11 | 2.12 | 2.80 | 0.79 |
| FLM 3 | 0.06 | 1.00 | 1.51 | 1.20 | 4.64 | 0.52 |
| FLM 2 | 2.24 | 0.18 | 1.40 | 0.44 | 2.18 | 1.46 |
| Maximum unknown impurity | 0.51 | 0.70 | 9.16 | 0.40 | 4.08 | 4.50 |
| Total impurity | 2.96 | 0.12 | 0.77 | 0.55 | 2.08 | 0.34 |
Stress testing
Stress testing helps to determine the stability of the molecule that is exposed to degradation under different conditions and to validate the stability-indicating power of the analytical methods used. In this study, the degradation profile of FLM/ tetrahydrozoline hydrochloride ophthalmic solution was monitored by applying stress-testing conditions mentioned above in Section 2.3. According to the results of stress-testing studies (Table 11), under thermal, acidic, alkaline, oxidative, and photolytic conditions, known impurities have not been observed except for alkaline hydrolysis, standing at 60 °C for 14 days. The most degradation occurred in oxidative degradation, and it was noted that 36.79% unknown impurity formed. It was seen that there was an increase in the unknown impurities in all degradations.
| Table 11: Results of stress-testing studies. | ||||||||
| Compound | Untreated sample | Hydrolysis | Oxidative degradation | Thermal degradation | Photolytic degradation | |||
| Acidic | Basic | |||||||
| 25 °C, 14th day | 60 °C, 14th day | 25 °C, 14th day | 60 °C, 14th day | |||||
| Deltamedrane | ND | ND | ND | ND | ND | ND | ND | ND |
| 1,2-Dihydrofluoro-metholone | ND | ND | ND | ND | ND | ND | ND | ND |
| Bromidrine | ND | ND | ND | ND | 0.12 | ND | ND | ND |
| FLM 3 | ND | ND | ND | ND | ND | ND | ND | ND |
| FLM 2 | ND | ND | ND | ND | ND | ND | ND | ND |
| Maximum unknown impurity | 0.13 | 0.17 | 0.57 | 0.16 | 0.74 | 36.79 | 0.15 | 0.14 |
| Total impurity (%) | 0.24 | 0.28 | 2.14 | 0.29 | 2.53 | 37.84 | 0.31 | 0.25 |
| Assay (%) | 100.54 | 99.76 | 99.02 | 100.48 | 97.82 | 61.35 | 100.76 | 100.39 |
| Stability-indicating detected; a Mass balance: assay + total impurities | ||||||||
Stabilitystability-indicating HPLC-related substances method was developed, validated, and used during analyses of stability samples of Fluorometholone/ tetrahydrozoline hydrochloride ophthalmic solution. The method was validated according to International Conference onHarmonization (ICH) guideline and considered simple, sensitive, selective, linear, precise, accurate, robust, andstable for the determination of FLM impurities in its pharmaceutical formulation. The proposed methods were validated and could be used for routine analysis in quality control laboratories. Many parameters, such as choosing column, adjusting pH, played a critical rolein the retention time of the related compounds and their resolution from each other. The sample has reached maximum degeneration in oxidation degradation and was found sensitive to acidic and basic hydrolysis at 60 °C. Under other degradation conditions, there was very little degradation. In fact, there was almost no decay under the light degradation. In the literature, it was seen that there was no reported stability-indicating method for the determination of fluorometholone impurities. So, this method could be a guide for the determination of impurities of all formulations containing fluorometholone.
This work was supported by the World Medicine Pharmaceutical Industry and Trade Inc. The authors are thankful to the company for providing the necessary instrumental facilities and chemicals to carry out the research work.
Supporting information: Supporting information accompanies this paper.
- Marcos-Escribano A, Bermejo FA, Bonde-Larsen AL, Retuerto JI. Chemoselective hydrogenation of 17α-hydroxy-6-methylen-pregna-4,9(11)-diene-3,20-dione. Synthesis of fluorometholone. Tetrahedron. 2009;65:8493–8496.
- Hemchand S, Babu RRC, Annapurna MM. Stability-indicating reversed-phase high-performance liquid chromatography method for the determination of fluorometholone in bulk and pharmaceutical formulation. Asian J Pharm. 2018;12:760–765. Available from: https://doi.org/10.22377/ajp.v12i02.2426
- Pawar AK, Mannepalli C. Stability indicating analytical HPLC method development and validation for the simultaneous quantification of tobramycin and fluorometholone in ophthalmic suspension. J Pharm Negat Results. 2023;14:910–917.
- Fluorometholone Eye Drops, British Pharmacopeia, 2025.
- Fluorometholone, Fluorometholone Ophthalmic Suspension, Neomycin Sulfate and Fluorometholone Ointment, US Pharmacopeia 43-NF38.
- Vanoosthuyze KE, Van Poucke LSG, Deloof ACA, Van Peteghem CH. Development of a high-performance thin-layer chromatographic method for the multi-screening analysis of corticosteroids. Anal Chim Acta. 1993;215:177–182. Available from: https://scispace.com/papers/development-of-a-high-performance-thin-layer-chromatographic-5b7d6celr5
- Vladimirov S, Cudina O, Agbaba D, Zivanov-Stakic D. Spectrophotometric determination of fluorometholone in pharmaceuticals using 1,4-dihydrazinophthalazine. Anal Lett. 1996;29(6):921–927. Available from: https://doi.org/10.1080/00032719608001444
- Saleh SS, Lotfy HM, Hassan NY, Elgizawy SM. A comparative study of validated spectrophotometric and TLC- spectrodensitometric methods for the determination of sodium cromoglicate and fluorometholone in ophthalmic solution. Saudi Pharm J. 2013;21(4):411-21. Available from: https://doi.org/10.1016/j.jsps.2012.11.001
- Sharma MV, Patel ND, Prajapati B, Shah SK. Development and validation of stability indicating RP-HPLC method for estimation of ketorolac and fluorometholone in ophthalmic formulations. Int J Pharm Sci Nanotechnol. 2017;10(5):3815–3826. Available from: https://doi.org/10.37285/ijpsn.2017.10.5.2
- International Conference on Harmonisation. Validation of analytical procedures: text and methodology Q2(R2). Geneva (CH): ICH; 2023.