More Information
Submitted: November 27, 2024 | Approved: August 01, 2025 | Published: August 04, 2025
How to cite this article: Eraky ME, Said HEM, Ahmed RZ. Laparoscopic Subtotal Cholecystectomy after Grade II or III Cholecystitis via Percutaneous Transhepatic draining of the Gallbladder. Arch Case Rep. 2025; 9(8): 245-253. Available from:
https://dx.doi.org/10.29328/journal.acr.1001153
DOI: 10.29328/journal.acr.1001153
Copyright license: © 2025 Eraky ME, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Subtotal cholecystectomy; Percutaneous transhepatic gallbladder drainage; Safety; Feasibility
Laparoscopic Subtotal Cholecystectomy after Grade II or III Cholecystitis via Percutaneous Transhepatic draining of the Gallbladder
Mohamed E Eraky1*, Huda EM Said2 and Rham Z Ahmed3
1Surgical Department, Faculty of Medicine, Zagazig University, Zagazig City, Egypt
2Clinical Pathology Department, Faculty of Medicine, Zagazig University, Zagazig City, Egypt
3Medical Department, Zagazig University, Zagazig City, Egypt
*Address for Correspondence: Mohamed E Eraky, Surgical Department, Faculty of Medicine, Zagazig University, Zagazig City, Egypt, Email: [email protected]
Background: Subtotal cholecystectomy is indicated in certain circumstances when it is challenging to identify the anatomical gallbladder structures or if there is a high risk of iatrogenic damage. For acute calculous cholecystitis, the 2020 World Journal of Emergency Surgery recommends subtotal cholecystectomy. Intraoperative cholangiography, conversion to open surgery, aborting the process, and subtotal cholecystectomy are examples of possible bail-out techniques that can be performed during problematic cholecystectomy, especially after percutaneous drainage. Subtotal cholecystectomy is considered the best backup plan when a critical view of safety cannot be achieved during a challenging cholecystectomy.
Objective: This study aimed to assess the effectiveness and accessibility of laparoscopic SC after PTGBD for Grade II or III acute cholecystitis patients.
Methods: The time frame for this retrospective study spans from December 2014 to January 2022. Based on the appearance of cholecystitis, 88 patients with degree II or III AC were divided into pre-TG18 (2014–2018, n = 34) and post-TG18 (2018–2022, n = 54) groups. We examined the patients’ demographic backgrounds, surgical methods, and postoperative outcomes.
Results: The duration of PTGBD was significantly longer in the post-TG18 group (15 [interquartile range–9-42] days vs. 8 [4-11] days). The prevalence of laparoscopic cholecystectomy significantly increased to 52.9% in the study population before TG18 and to 88.9% in the group after TG18 (p = 0.001), in contrast to the SC rates, which were 23.5% and 40.7%, respectively, and did not vary significantly (p = 0.241). Among the 15 SC patients, the proportion of laparoscopic SC patients increased from 0 to 90.9%, while the proportion of open SC patients decreased noticeably, dropping from 100 to 9.1% (p = 0.001). There were no appreciable changes in the length of the operation, quantity of intraoperative bleeding, or frequency of complications following surgery (subhepatic abscess and wound infection). There were no deaths, bile leaks, or bile duct injuries in any of the groups.
Conclusion: Strong support for SC enhanced the success rate of laparoscopic surgery for Grade II or III AC after PTGBD. Thus, laparoscopic SC is safe and practical.
Thus, there is an alternative option for urgent cholecystectomy in critically ill patients with significant cholecystitis. These patients often require immediate therapy, with procedures that are minimally facilitated, and patients are often diagnosed before ultrasound and tomography are used. Laparoscopic cholecystectomy before fibrosis is effective and safe for patients with acute cholecystitis 72–96 hours after symptom onset. However, for the majority of patients with Grade II,/III AC based on a white blood cell (WBC) count > 18,000, a palpable tender mass in the right upper abdominal quadrant, a period of inquiries > 72 h, and marked local pain (gangrenous cholecystitis, pericholecystic abscess, hepatic abscess, biliary peritonitis, and emphysematous cholecystitis), such treatment is uncertain, and it is challenging to predict potential consequences [1].
Patients with acute cholecystitis with multiple comorbidities, severe inflammation, and serious adhesions will undoubtedly have additional concerns and increase the possibility of conversion to open surgery.
For the treatment of severe or moderate Acute Cholecystitis (AC) in individuals with a deteriorated health status without recovery after antibiotics or supportive treatment, percutaneous transhepatic gallbladder drainage (PTGBD) and elective/delayed cholecystectomy are encouraged by the Tokyo Guidelines 2018 (TG18) [2]. Diminished cholecystectomies and postoperative hazards are critical issues because many PTGBD patients are elderly or have primary concurrent medical conditions [2,3]. Laparoscopic cholecystectomy (LC) for AC following PTGBD, however, is difficult to perform because of the significant adhesions to each other, contributing to almost significant open rates for conversion [4].
Due to significant adhesions between the liver and gallbladder wall, after grade II or III acute cholecystitis, percutaneous transhepatic gallbladder drainage, laparoscopic subtotal cholecystectomy was performed from the start of the Tokyo Guidelines 2018 (TG18), before the transition from subtotal cholecystectomy (SC) to open conversion. Total laparoscopic cholecystectomy is not an option for patients with complex cholecystitis.
Additionally, open conversion is unsafe for completing cholecystectomy, which makes the subtotal type more accepted [4,5]. The TG18 procedure is considered an intermittent stage until the acute-stage reaction subsides; therefore, patients’ health gains proceed to the surgery of choice [5].
Statistically significant disparities among the illnesses that were confirmed and those that were only suggested were revealed in a Japanese study that examined the relationship between diagnostic criteria and variables, including hospital stay time and medical costs [6], highlighting the value of these testing requirements. Given the findings of these two investigations, we concluded that the TG13 diagnostic criteria for acute cholecystitis do not present any significant issues and advise their continued use as the TG18/TG13 diagnostic guidelines (Table 1).
| Table 2: TG18/TG13 diagnostic criteria for acute cholecystitis |
| A. Imaging findings |
| Imaging findings characteristic of acute cholecystitis |
| Suspected diagnosis: one item in A + one item in B |
| Definite diagnosis: one item in A + one item in B + C |
| B. Local clinical inflammatory picture. |
| C. Systemic inflammatory syndrome |
According to the TG18, if the risk of total cholecystectomy is still considerable regardless of laparotomy, subtotal cholecystectomy (SC) is an appropriate surgery. The choice to undergo open conversion should be made after considering the practitioner’s knowledge and expertise [5]. BD patients are elderly people who may also have serious complications [2]. Laparoscopic cholecystectomy (LC) for AC following PTGBD is difficult to perform because of significant fibrosis and adhesion, resulting in high open conversion rates [3]. Additionally, open conversion is not always secure and cannot render total cholecystectomy simpler for practitioners lacking significant open cholecystectomy knowledge [4].
In our institution, when total LC is impractical, open conversion is chosen for complex AC cases. Since the release of the TG18, open conversion has been replaced with laparoscopic SC. Laparoscopic SC has recently received much attention, and studies have shown that this technique helps treat complicated cholecystitis [7]. However, the effectiveness and viability of laparoscopic SC following PTGBD remain unclear.
Using information gathered from a Zagazig University Hospital between Dec. 2014 and Jan. 2022, we conducted a retrospective cohort study.
Inclusion criteria
Individuals who had received PTGBD for cholecystectomy and had a grade of II or III, Fitness for surgery and nonmalignant or debilitating disease.
Exclusion criteria
Two patients had a cholecystocolonic fistula at the time of surgery, four patients underwent cholecystectomy for other cancers of the gut, and Unfitness or malignant debilitated comorbidity.
Sampling size
The results from the physical examination, sonography, and computed tomography were used to diagnose AC. TG18 was used to determine the grade (level of severity) of AC [7]. Based on variables such as age, duration of symptoms, associated comorbidities, and continued symptoms even with supportive treatment and antibiotics, physicians advised PTGBD.
Radiology investigation
US has been widely used to treat acute cholecystitis, and previous case series studies have described simple and noninvasive methods. However, the diagnostic yield mentioned in these articles varies depending on the tool, evaluation standards, and criteria for diagnosis applied in every study, all of which were performed on a limited number of people (one institution). Hepatobiliary scintigraphy (HIDA scanning) has a greater diagnostic yield than ultrasound (US) in each investigation, and three newly suggested standards still endorse testing with US.
The US is the most beneficial choice for the evaluation of acute cholecystitis because it is relatively inexpensive compared to other imaging modalities, including Computed Tomography (CT) and magnetic resonance imaging (MRI), and because it is noninvasive and has a high diagnostic yield. Its use in clinical practice has been reported to be 61.3% 7.
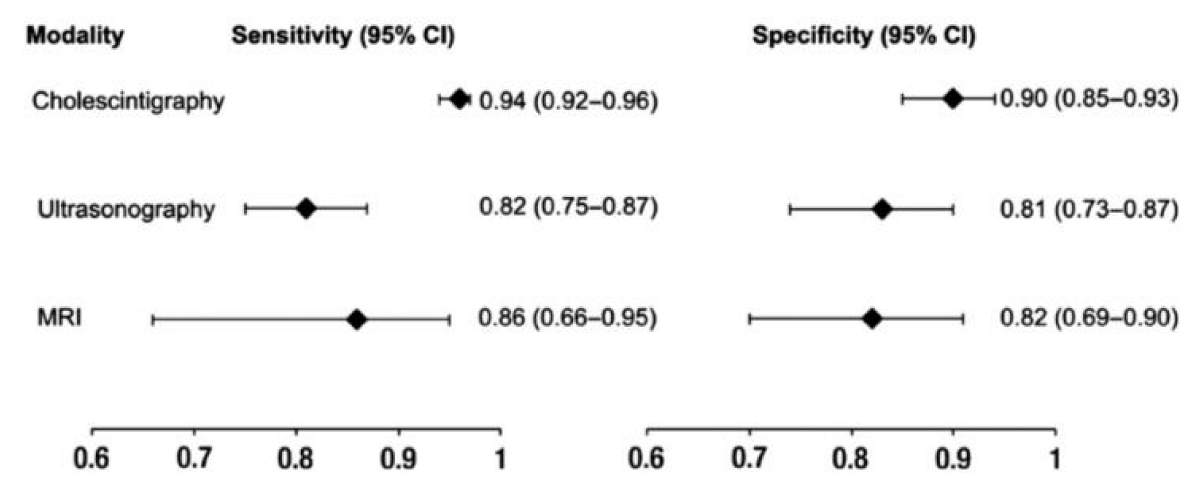
According to a meta-analysis evaluating several diagnostic imaging techniques for acute cholecystitis, ultrasound has 81% sensitivity (95% CI: 0.75-0.87) and 83% specificity (95% CI: 0.74-0.89) (Figure 1).
Figure 1: Meta-analysis evaluating by diagnostic imaging techniques for acute cholecystitis.
The surgeon decided on PTGBD for patients whose manifestations did not improve after receiving IV antibiotics, in addition to those whose complaints were assessed to indicate poor tolerance of emergency surgery according to age, time since the beginning, and the existence of concomitant conditions. A second comparative analysis was performed in the post-TG18 group to further understand the differences in severity and overall states between the direct cholecystectomy and post-PTGBD cholecystectomy groups. In accordance with the first manifestation of cholecystitis, the 44 patients included in the present investigation were split into two groups: the pre-TG18 group (2013–2017, n = 34) and the post–TG18 group (2018–2020, n = 54). The scientific characteristics, surgeries, immediate postoperative results, and complications following surgery in the two categories were compared. Wherever applicable, the research was published in accordance with STROBE’s qualitative research reporting criteria [8].
Medical supportive treatment of acute-stage disease
Incisive antibiotics were administered after culture and sensitivity, ICU admission, and treatment if the general condition deteriorated; excess IV fluids and antibiotics such as imipenem/cilastatin or meropenem were administered, and intravenous noradrenaline was used to stabilize blood pressure.
When acute cholecystitis was diagnosed, antibiotics and supportive intravenous fluid were administered. Wastment was initiated, for example, prophylactic sulbactam–ampicillin with fluid supplementation.
Management of unfit patients for PTGBD (temporary procedures)
Surgery was postponed after an improvement in the patient’s general condition. Cholecystoangiography can be performed through the PTGBD tube if the cystic duct is patent; after improvements in physical condition, cholecystectomy can be performed; if the cystic duct is patent, the PTGBD tube can be removed preoperatively, but if the cystic duct is obstructed, it can be removed intraoperatively, until the patient becomes fit for the operation.
U/S-guided intrahepatic gallbladder aspiration (with an 18-G needle) was performed with a fixed catheter (7- to 8-Fr pigtail through the guidewire) inside the gallbladder for continuous drainage under a fluoroscope.
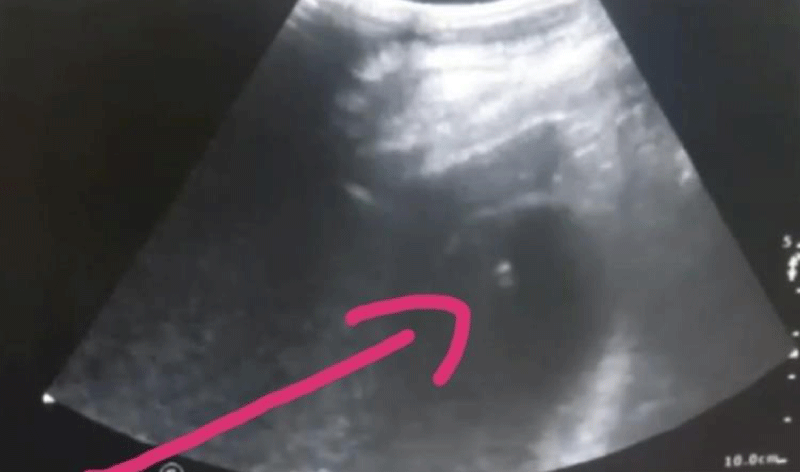
The gallbladder can be scanned using a PTGBD tube (cholecysto-cholangiography) (Figure 2).
Figure 2: PTGBD -guided PTGBD tube, U/S showing the gallbladder lumen catheter indicated by the arrow
Bailout surgery
In general, if the surgeon fails to identify the cystic artery or duct for one hour because dense fibrosis around Calot’s triangle indicates liver bleeding, duodenum, or CBD injury, the operation must be converted to open surgery to prevent CBD hazards, duodenal injury, or continuous hemorrhage.
Fundus-first surgery was indicated if there was dense fibrosis masking the field of the cystic duct and Hartmann pouch, or if there was distorted cystic duct dilatation. The stump was closed with 3/0 Prolein after ENDOLOOP®, or a ligature was used to ligate the dilated cystic duct. The gallbladder remnant must be treated with diathermy spray or LigaSure to prevent further mucocele or malignant complications.
Statistical analysis
For the statistical analyses, Stat View 5.0 (SAS Institute Inc., Cary, NC, USA) was used.
Investigation data were compared between the pre-TG18 and post-TG18 groups, and changes in surgical outcomes and treatment modalities were assessed via univariate analysis. Statistical analyses were two-sided, with a p - value of 0.05, which was considered indicative of statistical significance.
The data are presented as numbers and percentages (%) or interquartile ranges (IQRs) according to the field. The chi-squared test was used for categorical variables. The Mann‒Whitney U test was used for continuous variables. Surgical outcomes, clinical outcomes.
A retrospective cohort study using data collected from a single facility; acute cholecystitis was detected in 1090 patients between December 2014 and December 2022, and 544 patients underwent cholecystectomy. Ninety-four patients (17.2%) underwent PTGBD tube implantation.
Four patients who underwent cholecystectomy for other GUT carcinomas, and two patients with gall bladder and duodenal fistula (CDF) were excluded.
Moreover, in comparison, the severity and fitness of the two subcategories (n=208 individuals) were split into
∗ Receiving direct cholecystectomy treatment (n = 154) and
∗ Post-PTGBD cholecystectomy (n = 54).
The clinical characteristics between acute cholecystitis and PTGBD and between PTGBD drainage and cholecystostomy are shown in Table 2, and the differences between the two groups in terms of procedure outcomes are shown in Table 3.
| Table 2: Patient demographics and clinical presentations in the pre-TG18 group and post-TG18 group. | |||
| Variable | Pre-TG18 group (2014–2018, n = 34) | Post-TG18 group (2018–2022, n = 54) | p value |
| Age/years), median [IQR] | 75 [63–84] | 78 [68–87] | 0.682 |
| Gender male | 12 (35.3%) | 36 (66.7%) | 0.042* |
| Severity grade of AC | 0.234 | ||
| II | 32 (94.1%) | 44 (81.5%) | |
| III | 2 (5.9%) | 10 (18.5%) | |
| ASA-PS | 0.143 | ||
| I–II | 24 (70.6%) | 26 (48.1%) | |
| III or higher | 5 (29.4%) | 28 (51.9%) | |
| SIRS | 20 (58.8%) | 36 (66.7%) | 0.599 |
| WBC count on admission × 103/μL, median [IQR] | 13.1 [10.7–20.6] | 14.9 [11.9–19.7] | 0.736 |
| CCI at the time of hospitalization | 1 [0–2.5] | 2 [1–3] | 0.219 |
| Associated morbidity | |||
| Cardiac infarction | 10 (29.4%) | 12 (22.2%) | 0.592 |
| CHF | 2 (5.9%) | 8 (14.8%) | 0.363 |
| Peripheral vasculitis | 0 | 6 (11.1%) | 0.155 |
| CVD | 6 (17.6%) | 10 (18.5%) | 0.942 |
| Dementia | 6 (17.6%) | 12 (22.2%) | 0.714 |
| Renal Failure | 0 | 4 (7.4%) | 0.251 |
| D.M | 10 (29.4%) | 16 (29.6%) | 0.988 |
| Hemiplegia | 0 | 8 (14.8%) | 0.096 |
| CKD | 0 | 4 (7.4%) | 0.251 |
| Tumor mass | 2 (5.9%) | 4 (7.4%) | 0.845 |
| Lymphoma | 2 (5.9%) | 0 | 0.202 |
| Time from onset to PTGBD, days, median [IQR] | 3 [1–7] | 2 [1–3] | 0.126 |
| Time from PTGBD to cholecystectomy, days, median [IQR] | 8 [4–11] | 15 [9–42] | 0.010* |
| The 2018 Tokyo Guidelines, when appropriate, values are shown as n (%) or median [IQR, 25th and 75th percentiles] * p- p-values significant at (p < 0.05). | |||
| Table 3: Surgical types and results | |||
| Variables | Pre-TG18 group (2014–2018, n =34) | Post-TG18 group (2018–2022, n=54) | P value |
| Open/ /laparoscopic intervention | 16 (47.1%)/18 (52.9%) | 6 (11.1%)/48 (88.9%) | 0.007* |
| Open conversion rate | 6 (33.3%) | 6 (12.5%) | 0.167 |
| SC | 8 (23.5%) | 22 (40.7%) | 0.241 |
| Open SC/laparoscopic SC | 8 (100.0%)/0 | 2 (9.1%)/20 (90.9%) | 0.001* |
| Open conversion rate | 0 | 6 (30.0%) | |
| Operative time, min [IQR] | 137 [97–181] | 125 [111–148] | 0.373 |
| Blood loss, mL [IQR] | 110 [3–305] | 45 [15–100] | 0.353 |
| Hospitality time, days [IQR] | 10 [4–13] | 8 [4–20] | 0.515 |
| Incision infection | 2 (5.8%) | 2 (3.7%) | 0.736 |
| Subhepatic suppuration | 2 (5.8%) | 4 (7.4%) | 0.845 |
| Bile leak | 0 | 0 | |
| CBD injury | 0 | 0 |
|
| The 2018 Tokyo Guidelines When appropriate, values are shown as the n (%) or median [IQR, 25th and 75th percentiles] * p - values significant at (p < 0.05. |
|||
AC grade II or III after TG18 is shown in Table 1S The patients in the post-PTGBD cholecystectomy group were significantly older, had more severe disease, and had more comorbidities.
| Table 1S: Clinical presentation of the direct cholecystectomy and post-PTGBD cholecystectomy groups after TG18. | |||
| Variable | Direct cholecystectomy group (n = 154) | Post-PTGBD cholecystectomy group (n = 54) | P - value |
| Age /years), median [IQR] | 65 [49–76] | 78 [68–87] | < 0.001* |
| Gender males | 60 (39.0%) | 36 (66.7%) | 0.013* |
| Severity grade of AC | 0.001* | ||
| II | 152 (98.7%) | 44 (81.5%) | |
| III | 2 (1.3%) | 10 (18.5%) | |
| ASA-PS | 0.006* | ||
| I – II | 118 (76.6%) | 26 (48.1%) | |
| III or higher | 36 (23.4%) | 28 (51.9%) | |
| SIRS | 62 (40.3%) | 36 (66.7%) | 0.018* |
| CCI on admission, median [IQR] | 0 [0–2] | 2 [1 –3] | 0.001* |
| The 2018 Tokyo Guidelines, when appropriate, values are shown as n (%) or median [IQR, 25th and 75th percentiles] * p - values significant at (p < 0.05). | |||
More comorbidities were associated with aging progression, post-PTGBD cholecystectomy, systemic inflammatory response syndrome, white blood cell count on admission, and the Charlson Comorbidity Index. There were significantly more men in the post-TG18 group (p = 0.042). The American Society of Anesthesiologists physical status (ASA-PS) class I–II was lower in the post-TG18 group (70.6% vs. 48.1%), and there were no differences at the time of admission between the groups in age, severity of attack, grading of the AC or SIRS to infection, Charlson Comorbidity index, or WBC count.
The post-TG18 group included significantly more men (p = 0.042). The post-TG18 group had a greater percentage of class III (29.4% vs. 51.9%, p = 0.143) and a decreased percentage of ASA-PS classes I-II (70.6% vs. 48.1%). However, this difference was not significant.
The median distance between cholecystitis onset and PTGBD introduction in the pre-TG18 group was three days (IQR: 1–7). and 2 days post-TG-18 treatment (IRQ:1-3), but the difference was not significant (p = 0.126).
The median duration from the beginning of PTGBD to cholecystectomy increased significantly in the post-TG18 group, from 8 (IQR: 4-11) days in the pre-TG18 group to 15 (IQR: 9-42) days (p = 0.010). Table 3: Lines of surgery and results. The proportion of LC increased significantly from 52.9% in the pre-TG18 group to 88.9% in the post-TG18 group (p = 0.007). Among the 88 patients who underwent SC, 23.5% were in the pre-TG-8 group, and 40.7% were in the post-TG-18 group (p = 0.241); these differences were not significant. Moreover, among the thirty SC patients, the percentage of laparoscopic SC patients increased significantly from 0 to 90.9% (p = 0.001), but the percentage of open SC patients sharply declined considerably from 100 to 9.1% (p = 0.001).
There were significant differences between the groups in terms of the length of the procedure, intraoperative bleeding, hospitalization time, and incidence of wound, intraabdominal, subhepatic or diaphragmatic infection. However, in the post-TG18 group, the operation duration decreased (137 vs. 127 min), bleeding decreased, the amount of blood (110 mL) decreased to 45 mL, and hospitalization decreased from 10 days to 8 days. No cases of CBD injury, other sources of bile leakage or fatality were detected.
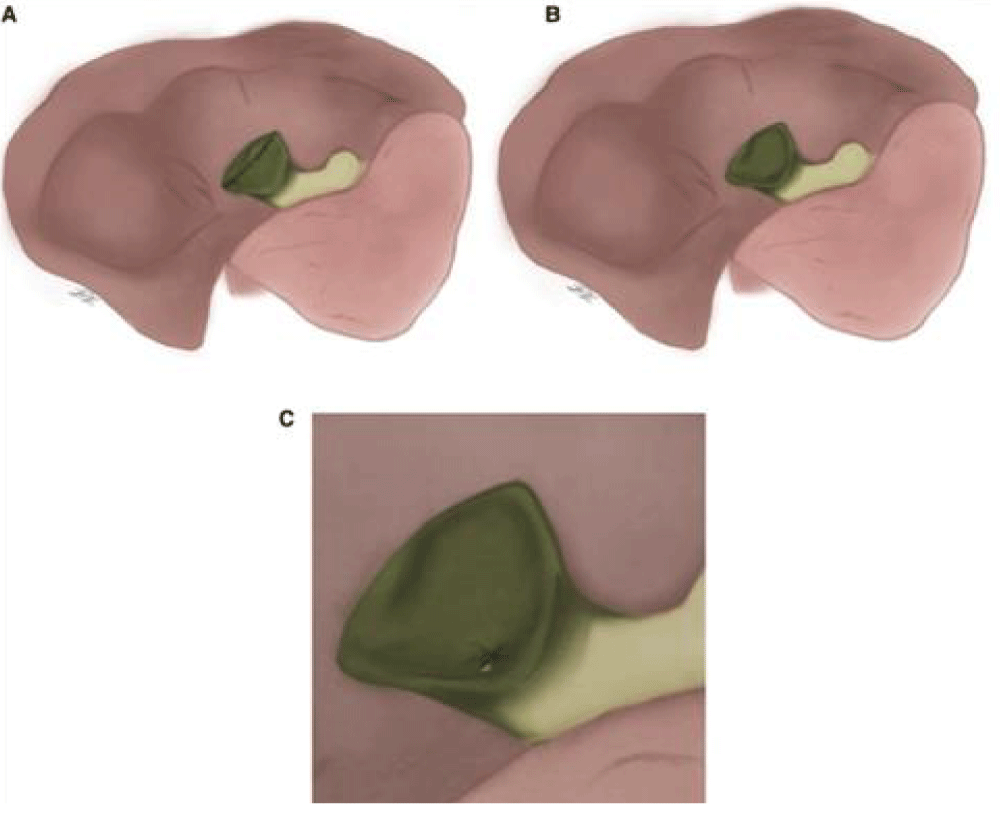
Table 4 lists 30 SC cases. Surgical intervention for Henneman type B SC occurred in 26 patients (86.7%). After meticulous dissection of the retained part of the bladder posterior wall, the cystic duct and cystic artery were ligated (Figure 3) [9].
| Table 4: Results of subtotal cholecystectomy | ||||||||
| Case no | Group | Mirizzi syndrome | Lap/open conversion | Fundus-first surgery | Types of subtotal cholecystectomy | Wound infection | Subhepatic suppuration | |
| Henneman division | Strasberg division | |||||||
| 1 | Pre-TG18 | − | Open | − | Type B | − | − | |
| 2 | Pre-TG18 | − | Open | − | Type B | − | − | |
| 3 | Pre-TG18 | − | Open | − | Type B | − | + | |
| 4 | Pre-TG18 | − | Open | − | Type B | − | − | |
| 5 | Post-TG18 | − | Conversion | + | Reconstituting type A | + | − | |
| 6 | Post-TG18 | − | Lap | + | Type B | − | − | |
| 7 | Post-TG18 | − | Lap | − | Type B | − | − | |
| 8 | Post-TG18 | − | Lap | − | Type B | − | − | |
| 9 | Post-TG18 | − | Conversion | + | Type B | − | − | |
| 10 | Post-TG18 | − | Lap | − | Type B | − | − | |
| 11 | Post-TG18 | − | Lap | − | Type B | − | − | |
| 12 | Post-TG18 | − | Lap | + | Type B | − | − | |
| 13 | Post-TG18 | + | Conversion | − | Reconstituting type A | − | + | |
| 14 | Post-TG18 | − | Open | − | Type B | − | − | |
| 15 | Post-TG18 | − | Lap | + | Type B | − | − | |
| TG18 Tokyo Guidelines 2018 | ||||||||
Figure 3: (Strasberg) Reconstruction type; (A) fenestrate type; (B ). Fenestrating type with internal cystic duct suture closure. (C) [20]
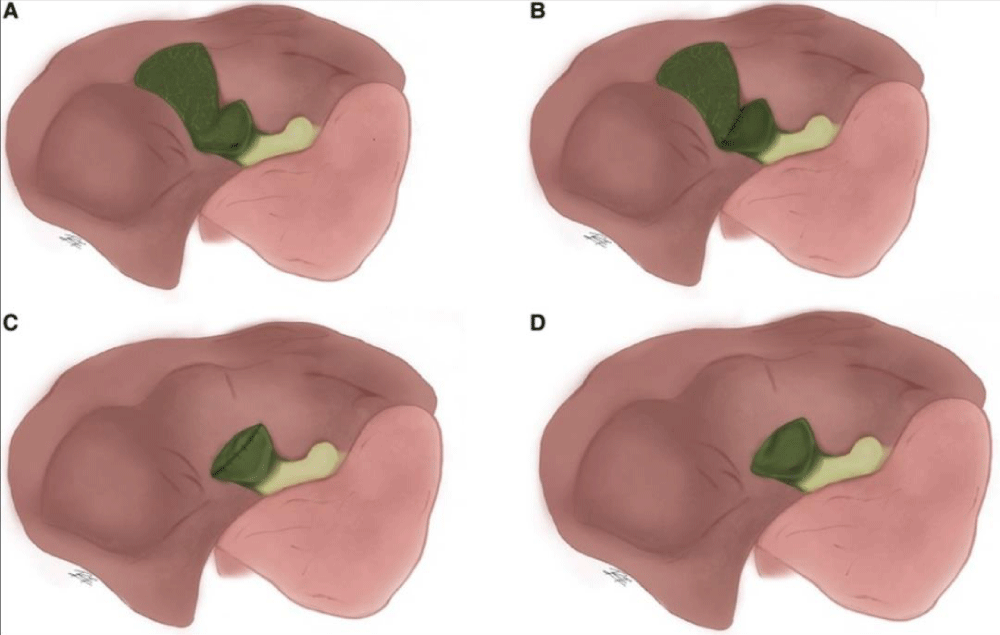
The final four patients (13.3%) underwent a different SC procedure called Strasberg reconstituting type A, which preserves a piece of the posterior wall of the gallbladder after suturing and sealing the infundibulum stump (Figure 4) [10]. A patient with Mirizzi syndrome has been reported.
Figure 4: Subtotal cholecystectomy according to Henneman. (A) Departing the portion of the gallbladder stumps that connects to the liver exposed and the rest of the gallbladder stumps open; (B) performing type A surgery, but with the gallbladder stump closed; (C) resecting both the anterior and posterior gallbladder walls, with the entire stump closed; and (D) performing type C surgery, but with the gallbladder stumps exposed [20].
Grouping
Subtotal cholecystectomies were classified as “fenestrating” or “reconstituting” by Strasberg, et al. according to whether the remaining gallbladder was left open or closed (Figure 3). It is possible to perform a fenestrating subtotal cholecystectomy with or without internal cystic duct suture closure. The gallbladder section that comes into contact with the liver in both cases might be removed or left in place. In an effort to prevent biliary fistulas, “reconstituting” type cholecystectomies are carried out; however, they increase the chance of gallstone formation in the remaining gallbladder and, as a result, the likelihood of recurrent biliary illness [5].
Henneman proposed a more comprehensive classification scheme that divides subtotal cholecystectomy into four methods: (A) resection of the anterior and posterior gallbladder walls with the stump closed, without placing a drain; (B) similar to type A but with the gallbladder stump closed; (C) resection of both the posterior and posterior gallbladder walls with the stump closed; and (D) similar to C but with the gallbladder stump open, placing a drain (Figure 4) [8,11].
The current investigation involved patients who underwent PTGBD and had grade II or III AC. This study revealed that the post-TG18 group had significantly greater laparoscopic surgery completion rates and laparoscopic SC rates, which lengthened the time between PTGBD and cholecystectomy. Although significant variations in procedural approaches and treatment plans were found, they had no adverse effects on the surgical results.
Significant fibrosis and gallbladder adhesion may lead to technical difficulties and postoperative complications for some patients who undergo LC after PTGBD [3,12].
The complexity of a cholecystectomy can be estimated preoperatively from clinical, radiologic, and laboratory data, but it is not visible until after surgery [13].
The likelihood of completing a subtotal cholecystectomy is directly correlated with the number of preoperative variables for difficult cholecystectomy [14]. We can thus conclude that factors associated with difficult cholecystectomy are comparable to those associated with partial cholecystectomy because this procedure is one of the therapy choices for difficult cholecystectomy.
A number of studies reported the risk factors have been linked to subtotal cholecystectomy, including male sex (OR = 2.59 (7)), older age (OR = 1.23 (7)), ASA score of ≥3 (OR = 3.84) (15), white blood cells (WBC) (OR = 2.02 (7), albumin level (OR = 0.31 16), preoperative diagnosis of acute on chronic cholecystitis (OR = 5.47 (7)), acute cholecystitis (OR = 2.69 16), a higher Tokyo grade for severity of acute cholecystitis (OR = 2.37 17), a history of liver disease (OR = 8.40) (16), the amount of time before surgery (OR = 5.31 15), and previous biliary tract drainage (cholecystostomy) (OR = 2.66 (7,9,16, 18)). There have also been documented image findings, including disruption of the common hepatic duct (OR = 3.92) (15) and obscuration of the gallbladder wall around the neck (OR = 10.56) (15).
Inflammation in the surgical setting can cause bleeding. Moreover, significant adhesion and scarring make it challenging to distinguish the anatomical parts [15]. Other researchers have focused on the time interval between PTGBD and postoperative complications to determine the ideal time for cholecystectomy after PTGBD, but no sharp distinction has been reached [13].
However, recent studies [15-17] advocate delaying cholecystectomy. According to Hye, et al., patients who underwent cholecystectomy at least two weeks after the PTGBD procedure had shorter operation times, fewer postoperative problems, and less need for postoperative hospitalization. In the study by Sakamoto, et al., the ideal time for surgery following PTGBD was an average of 7-26 days [14]. Inoue, et al.’s study of 67 patients who underwent cholecystectomy within nine days of PTGBD showed an increased likelihood of postoperative complications [18].
One advantage of delaying cholecystectomy, along with stabilizing the patient’s health status, is the second subside of acute inflammatory stages and tissue infections. The patients in the post-TG18 group in our study had a significantly longer period between PTGBD and the procedure, and with increasing age, the severity of an acute attack of cholecystitis worsened, so the ASA-PS worsened, and the patients in the post-TG8 group had a greater risk and needed more time to stabilize. Cholecystectomy was performed as often as possible during the same hospitalization after PTGBD in both periods in the same facility, when the patient’s overall condition stabilized.
Post-TG18 patients showed a trend towards shorter or decreased median operating times, blood loss, and postoperative hospital stay without increased postoperative complications.
According to our research, delaying cholecystectomy may result in better surgical results, even in individuals in critical condition.
In our study, the introduction of SC after TG8 resulted in a significant change in the composition of patients who underwent cholecystectomy.
Subtotal cholecystectomy is a safe alternative for individuals with difficult cases of Callot’s triangle. Gallbladder percutaneous transhepatic drainage is recommended for elderly patients with concomitant conditions who are not in good overall health. Without requiring surgery, radiography can be used to assess whether the ductus cysticus is open in very elderly patients after the catheter is removed. Callot’s triangle makes it simple to perform a total cholecystectomy on any patient who has decided to undergo surgery. Based on my own experience, most of these patients need to undergo laparoscopic or open total cholecystectomy. Although it is rarely necessary, laparoscopic partial cholecystectomy can be performed in the event of a technical issue.
[9,10], Horiuchi, et al. demonstrated that SC decreased surgical duration, bleeding, hospitalization, and the conversion rate, especially in patients who had more adherent gallbladder walls [19].
When 168 patients were studied for SC, Wee, et al. reported no CBD injury or 30-day mortality [20]. Following SC, bile leakage occurs 10% - 18% of the time [9,17,20]. More postoperative bile leakage was reported with SC than with open cholecystectomy, but less frequent injury of the bile ducts, postoperative complications, reoperation, and death were reported [20-22]. We did not observe bile leakage in 30 patients with SC because the cystic duct was always tied off, and the residual gallbladder stump was closed.
Strasberg’s and Henneman’s classification are unclear and not understabdable. This classification has changed over time. Lastly, subtype 3 is for cases with extensive adhesions and inflammation, in which the gallbladder is fenestrated high up on the fundus and gallstones are evacuated while leaving the remnant gallbladder open. Purzner, et al. establish five subtypes of subtotal cholecystectomy depending on whether the gallbladder stump is closed or not, whether the gallbladder portion attached to the liver is resected or left as is, and finally, based on these factors. This kind also includes damage-control cholecystectomy, which is limited to cases in which the gallbladder cannot be fully exposed due to aggressive adhesions [2].
Henneman divided SCs into four types (A, B, C, and D). In contrast, Strasberg divided SCs into fenestration and reconstituting types, based on differences in the posterior wall and infundibulum management [9,17,20].
Henneman’s analysis of the four degrees of SC revealed that Type B SC had no comorbidities, such as resurgery, bile leakage, or ERCP injury [9]. In the current study, 26 (86.7%) of the 30 individuals experienced Henneman type B SC, requiring closure of the cystic artery and duct with remnants of the gallbladder wall (Figure 3).
The first option preserves the posterior gallbladder wall with the Hartmann’s pouch closed. The second option involves fully mobilising the posterior gallbladder wall off the liver bed. The third option preserves the posterior gallbladder wall but leaves the remnant open.
Lunevicius presents a classification scheme that differs from earlier ones. Previous categories sought to differentiate between different conceivable forms of gallbladder resection, while other classifications attempted to subdivide the kind of closure on subtotal cholecystectomy [20].
Last but not least, Lunevicius recently suggested substituting the phrases subtotal open-tract and subtotal closed-tract cholecystectomy for “fenestrating” and “reconstituting” subtotal cholecystectomy, as the former lacks specificity [2].
The results indicated that the dense fibrosis and distortion of the anatomy of Calot’s triangle made the operation difficult, resulting from repeated punctures and aspirations from the gallbladder in AC patients who underwent PTGBD type B SC surgery performed by Henneman, which was safer for the completion of laparoscopic cholecystectomy even after PTGBD.
In our study, meticulous dissection of Calot’s triangle and bile duct ligation were applied even after PTGBD, and we demonstrated that ligature and dissection of the cystic artery and ducts are often feasible following PTGBD. The majority of surgeons are inclined to perform a complete cholecystectomy under these circumstances. The posterior wall of the gallbladder remains difficult to separate. Despite this, the situation might not be much improved, and certain experts might ultimately choose to perform total cholecystectomy in an open surgical fashion. Following total cholecystectomy, surgery can result in modest biliary leakage, greater hemorrhage, and damage to the liver.
The results of our investigation and the backgrounds of our patients must be taken into consideration.
Our case study group was slightly older than those in other studies, which restricted its applicability to the general population [3,15,13,1518,23-25]. In our opinion, adopting SC after PTGBD may lead to better surgical outcomes than open conversion, especially in older and unfit individuals.
Prior to treatment, it is crucial to evaluate the likelihood of having a challenging cholecystectomy that could turn into a subtotal cholecystectomy using the tools of requesting assistance, setting up a suitable timetable for the operation, and telling relatives about it. Since this danger is not based on one aspect alone, instead of the total of several factors, several distinct scores that can potentially anticipate this danger have been developed (14, 42–45).
This study is subject to limitations. First, only a limited group of patients from one institute was included. Second, as the study was retrospective, it is possible that the information obtained may have been inaccurate or lacking. Third, there could be some differences in the indications for PTGBD among the various surgeons.
According to our research, postponing cholecystectomy following PTGBD can improve therapeutic results while reducing complications. SC has improved the success rate of laparoscopic surgery. However, SC increases the completion rate of laparoscopic surgeries.
After PTGBD cholecystectomy, laparoscopic SC is a safe and practical therapeutic option for Grade II or higher AC.
Declaration
Ethical approval and consent to participate: The study was approved by the Ethics Committee of Zagazig University medical Hospital , but informed consent needed to be waived for three reasons: First, the study lasted a long time; Second, it does not involve patient privacy information; In addition, this was a retrospective study and it did not interfere with treatment decisions, according to national regulations, and with the Declaration of Helsinki.
Availability of database and materials: The database used and or analysis is available with the corresponding author upon reasonable request. All the authors have shared and approved the database and final version.
Author contributions
HS, RA: contributed to the conception and design.
ME: organized the database and performed the statistical analysis.
ME: wrote sections of the manuscript and prepared the tables.
RA: contributed to the manuscript revision and investigation.
All the authors read, approved, and equally shared the submitted version.
- Purzner RH, Ho KB, Al-Sukhni E, Jayaraman S. Safe laparoscopic partial cholecystectomy in the face of significant cystohepatic triangle inflammation: a retrospective analysis and indicated therapy strategy for the troublesome gallbladder. Can J Surg. 2019;62(6):402–11. Available from: https://doi.org/10.1503/cjs.014617
- Lunevicius R. Review of the literature on partial resections of the gallbladder, 1898–2022: the outline of the conception of subtotal cholecystectomy and a suggestion to use the terms ‘subtotal open-tract cholecystectomy’ and ‘subtotal closed-tract cholecystectomy’. J Clin Med. 2023;12(3):1230. Available from: https://doi.org/10.3390/jcm12031230
- Maehira H, Kawasaki M, Itoh A, Ogawa M, Mizumura N, Toyoda S, et al. Prediction of difficult laparoscopic cholecystectomy for acute cholecystitis. J Surg Res. 2017;216:143–8. Available from: https://doi.org/10.1016/j.jss.2017.05.008
- Gupta C, Prousalidis J, Tzardinoglou E, Michalopoulos A, Fahandidis E, Apostolidis S, et al. Cholecystectomy with partial resection. HPB Surg. 1996;9:133–6.
- Strasberg SM, Pucci MJ, Deziel DJ, Brunt LM. Subtotal cholecystectomy ‘fenestration’ vs ‘reconstituting’ subtypes and the prevention of bile duct injury: definition of the optimal procedure in difficult operative conditions. J Am Coll Surg. 2016 Jan;222(1):89–96. Available from: https://doi.org/10.1016/j.jamcollsurg.2015.09.019
- Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: Acute cholecystitis diagnostic criteria and severity grading (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):41–54. Available from: https://doi.org/10.1002/jhbp.515
- Sabour AF, Matsushima K, Love BE, Alicuben ET, Schellenberg MA, Inaba K, et al. Trends in the usage of subtotal cholecystectomy for acute cholecystitis across the country. Surgery. 2020;167(5):569–74. Available from: https://doi.org/10.1016/j.surg.2019.11.004
- Toro A, Teodoro M, Khan M, Schembari E, Di Saverio S, Catena F, et al. Subtotal cholecystectomy for difficult acute cholecystitis: how to finalize safely by laparoscopy—a systematic review. World J Emerg Surg. 2021;16(1):45. Available from: https://doi.org/10.1186/s13017-021-00392-x
- Henneman D, da Costa BC, Vrouenraets BA, van Wagensveld SM, Lagarde S. A systematic study of laparoscopic partial cholecystectomy for the troublesome gallbladder. Surg Endosc. 2013;27(2):351–8. Available from: https://doi.org/10.1007/s00464-012-2458-2
- Strasberg SM, Pucci MJ, Brunt LM, Deziel DJ. Subtotal cholecystectomy "fenestration" vs. "reconstituting" subtypes and bile duct injury prevention: defining the appropriate approach in difficult surgical settings. J Am Coll Surg. 2016;222(1):89–96. Available from: https://doi.org/10.1016/j.jamcollsurg.2015.09.019
- Henneman D, Da Costa DW, Vrouenraets BC, Van Wagensveld BA, Lagarde SM. Laparoscopic partial cholecystectomy for the difficult gallbladder: a systematic review. Surg Endosc. 2013;27(2):351–8. Available from: https://doi.org/10.1007/s00464-012-2458-2
- Habib FA, Kolachalam RB, Khilnani R, Preventza O, Mittal VK. Laparoscopic cholecystectomy's use in the treatment of gangrenous cholecystitis. Am J Surg. 2001;181(1):71–5.
Available from: https://doi.org/10.1016/s0002-9610(00)00525-0 - Yamada K, Yamashita Y, Yamada T, Takeno S, Noritomi T. Asian J Surg. 2021;44:334–8.
- Yamada K, Yamashita Y, Yamada T, Takeno S, Noritomi T. Optimal timing for performing percutaneous transhepatic gallbladder drainage and subsequent cholecystectomy for better management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2015;22(12):855–61. Available from: https://doi.org/10.1002/jhbp.294
- Jeon HW, Jung KU, Lee MY, Hong HP, Shin JH, Lee SR. Surgical outcomes of percutaneous transhepatic gallbladder drainage in acute cholecystitis grade II patients according to time of surgery. Asian J Surg. 2021;44(1):334–8.Available from: https://doi.org/10.1016/j.asjsur.2020.08.008
- Inoue K, Ueno T, Nishina O, Douchi D, Shima K, Goto S, et al. For severe cholecystitis, the optimal timing of cholecystectomy is after percutaneous gallbladder draining. BMC Gastroenterol. 2017;17(1):1–7.
Available from: https://doi.org/10.1186/s12876-017-0631-8 - In patients with difficult acute cholecystitis, the effect of delayed laparoscopic cholecystectomy after percutaneous transhepatic gallbladder draining of Surgery. 2001;181:71-5.
- Tsukamoto M, Fujiogi H, Matsui H, Fushimi K, Yasunaga H. A nationwide inpatient database study on the timing of cholecystectomy after percutaneous transhepatic gallbladder draining for acute cholecystitis. J Hepatobiliary Pancreat Sci. 2020;22:920–6.
- Ie M, Katsura M, Kanda Y, Kato T, Sunagawa K, Mototake H. Laparoscopic subtotal cholecystectomy after percutaneous transhepatic gallbladder drainage for grade II or III acute cholecystitis. BMC Surg. 2021;21:1–7.
Available from: https://doi.org/10.1186/s12893-021-01387-w - Lunevicius R. Laparoscopic subtotal cholecystectomy: a classification, which encompasses the variants, technical modalities, and extent of resection of the gallbladder. Ann R Coll Surg Engl. 2020;102:315–7.
Available from: https://doi.org/10.1308/rcsann.2020.0007 - Purzner RH, Ho KB, Al-Sukhni E, Jayaraman S. Safe laparoscopic subtotal cholecystectomy in the face of severe inflammation in the cystohepatic triangle: a retrospective review and proposed management. Can J Surg. 2019;62(6):402–11. Available from: https://doi.org/10.1503/cjs.016818
- Doi K, Sato S, Yukumi M, Yoshida M, et al. In acute cholecystitis with significant fibrotic adhesions, a delayed laparoscopic subtotal cholecystectomy was performed. Surg Endosc. 2008;22:2720–3.
Available from: https://doi.org/10.1007/s00464-008-0010-6 - Philips JA, Lawes DA, Cook AJ, Arulampalam TH, Zaborsky A, Menzies D, et al. For difficult cholelithiasis, laparoscopic partial cholecystectomy is used. Surg Endosc. 2008;22:1697–700.
Available from: https://doi.org/10.1007/s00464-008-9906-2 - Hubert C, Annet L, Beers E, van, Gigot JF. For safe surgery in severe cholecystitis, the "inside approach of the gallbladder" is an alternative to the standard Calot's triangle dissection. Surg Endosc. 2010;24:2626–32.
Available from: http://dx.doi.org/10.1007/s00464-010-0966-5 - Kuwabara J, Watanabe Y, Kameoka K, Horiuchi A, Sato K, Yukumi S, et al. The utility of laparoscopic partial cholecystectomy with surgical cholangiography in the treatment of severe cholecystitis. Surg Today. 2014;44:462–5.
Available from: https://doi.org/10.1007/s00595-013-0545-2