More Information
Submitted: January 06, 2024 | Approved: January 18, 2024 | Published: January 19, 2024
How to cite this article: Limaiem F, Saffar K, Halouani A. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024; 8: 010-013.
DOI: 10.29328/journal.acr.1001087
Copyright License: © 2024 Limaiem F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Malignant germ cell tumor; Ovary; Surgery; Pathology; Immunohistochemistry
Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor
Faten Limaiem1,2 *, Khalil Saffar1,3 and Ahmed Halouani1,3
*, Khalil Saffar1,3 and Ahmed Halouani1,3
1University of Tunis El Manar, Tunis Faculty of Medicine, 1007, Tunis, Tunisia
2Pathology Department, University Hospital Mongi Slim La Marsa, Tunisia
3Gynecology Department, University Hospital Mongi Slim La Marsa, Tunisia
*Address for Correspondence: Faten Limaiem, Department of Pathology, Mongi Slim Hospital, La Marsa, Tunisia, Email: [email protected]
Background: Dysgerminomas, account for only 1% - 2% of all malignant ovarian neoplasms.
Objective: This case report aims to present a rare occurrence of dysgerminoma in a pediatric patient, highlighting the clinical characteristics, diagnosis, and management.
Case presentation: We present a case of dysgerminoma in a 12-year-old girl who presented with a three-week history of pelvic pain and progressive abdominal swelling. Physical examination revealed a distended abdomen with evident suprapubic fullness, and a palpable abdominopelvic mass measuring approximately 20 weeks in size. Imaging studies confirmed the presence of a solid heterogeneous mass originating from the pelvis. The patient underwent a right salpingo-oophorectomy without complications. Histological examination coupled with an immunohistochemical study confirmed the diagnosis of dysgerminoma. The patient had an uneventful postoperative course and was discharged without adjuvant treatment. Regular follow-up visits, physical examinations, ultrasound scans, and lactate dehydrogenase assays were initiated for monitoring.
Conclusion: Prompt recognition and appropriate surgical intervention, followed by regular monitoring, are crucial for optimal outcomes in pediatric dysgerminoma cases.
Dysgerminomas, the most common malignant primitive germ cell tumors of the ovary, account for only 1% - 2% of all malignant ovarian neoplasms [1]. These tumors primarily affect young women, but their incidence in the pediatric population is not well-documented, with only rare case reports found in pediatric literature [2]. Clinical manifestations commonly observed in this condition encompass abdominal pain, abdominal distention, the presence of a palpable mass, reduced appetite, as well as symptoms like nausea and vomiting [3]. Due to the insidious nature of the symptoms, the tumor is usually detected at an advanced stage during diagnosis. Treatment approaches that have shown effectiveness involve conservative surgery, chemotherapy, and postoperative radiation [3-5].
In this paper, we present a new case of ovarian dysgerminoma in a 12-year-old child, aiming to provide a comprehensive understanding of the clinical and pathological features associated with this rare neoplasm.
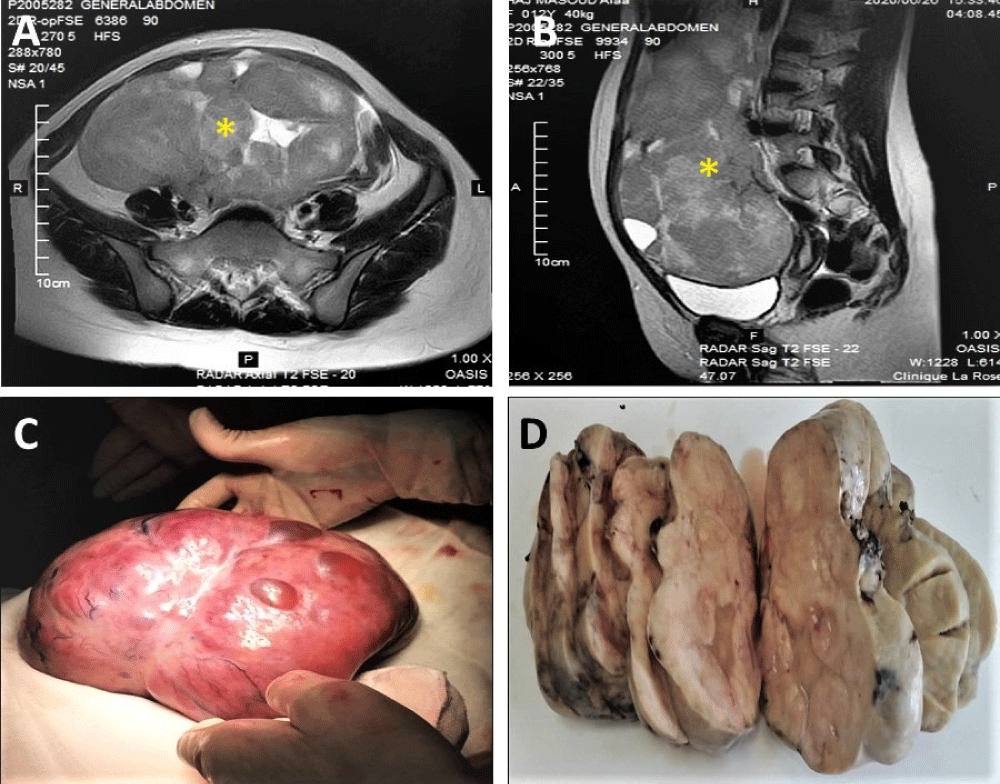
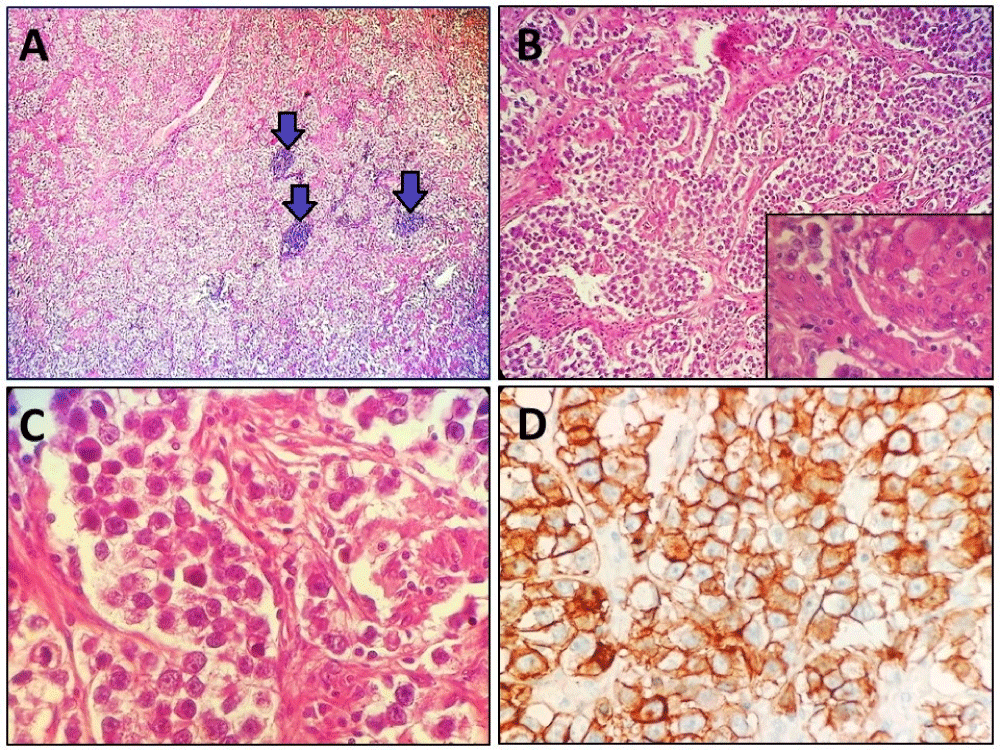
A 12-year-old girl, with a family history of renal cancer, presented to the gynecological oncology clinic with a three-week history of pelvic pain and progressive abdominal swelling. The pain was described as insidious, intermittent, and dull, without significant interference in her daily activities or sleep. Physical examination revealed a distended abdomen with evident suprapubic fullness, and a palpable abdominopelvic mass measuring approximately 20 weeks in size. The mass was characterized as firm, non-tender, smooth, and mobile. While tumor markers, including beta-human chorionic gonadotrophin and alpha-fetoprotein (1.1 U/ml), were within normal ranges, there was an elevation in Lactate Dehydrogenase (LDH) levels (380 U/l) and a discrete increase in cancer antigen 125 (CA 125) (82 U/ml). Both ultrasonography and abdominopelvic MRI performed on presentation revealed a large, solid, heterogeneous mass originating from the pelvis and extending superiorly into the abdomen (Figure 1A & 1B). The mass exhibited predominantly solid features with some areas of cystic degeneration. Measuring 20 cm × 16 cm × 8 cm, it appeared inseparable from the right ovary, which was not visualized. Subsequently, the patient underwent laparotomy and unilateral right adnexectomy (salpingo-oophorectomy). Intraoperative findings exhibited a large solid mass (20 cm × 16 cm × 8 cm) originating from the right ovary, with the right fallopian tube appearing grossly normal (Figure 1C). The contralateral ovary, fallopian tube, and omentum were all macroscopically normal. Intraoperative frozen section analysis of the tumor raised suspicion of an undifferentiated malignant neoplasm. Macroscopically, the ovarian tumor displayed clear delineation and encapsulation, with a solid, fleshy whitish appearance and areas of cystic degeneration (Figure 1D). Histological examination revealed a proliferation of tumor cells arranged in sheets, nests, and trabeculae, separated by collagenous septa containing lymphocytes (Figure 2A), and focal presence of sarcoid-like granulomas (Figure 2B). The tumor cells exhibited uniform features, including medium-sized nuclei with vesicular chromatin and prominent nucleoli (Figures 2C & 2D), along with a high number of mitotic figures. Immunohistochemical analysis demonstrated positive immunostaining of the tumor cells for CD117 and cytokeratin, while CD30 staining was negative. The final pathological diagnosis confirmed ovarian dysgerminoma. Omental biopsy and peritoneal fluid aspiration yielded negative results for malignancy. The patient had an uneventful postoperative course and was discharged on the fifth day after surgery. Currently, she is being closely monitored through regular follow-up visits, physical examinations, ultrasound scans, and LDH assays.
Figure 1: A & B: Magnetic resonance imaging showing a solid heterogeneous mass measuring 20 cm × 16 cm × 8 cm, arising from the pelvis and extending superiorly into the abdomen (Asterisk). The mass was mainly solid with some cystic areas within it. C: Per-operative aspect of the ovarian mass. D: Macroscopically, the ovarian tumor was well delineated and encapsulated. On the cut section, it was solid, fleshy whitish with focal foci of cystic degeneration.
Figure 2: A: Tumor proliferation arranged in trabeculae and sheets separated by fibrous septa containing lymphoid aggregates (arrows) (Hematoxylin and eosin, magnification × 40). B: Tumor proliferation arranged in trabeculae and sheets separated by fibrous septa (Hematoxylin and eosin, magnification × 100). Inset: Presence of a granuloma in the tumor proliferation (Hematoxylin and eosin, magnification × 400). C: Higher power image showing polygonal cells with distinct cell borders and eosinophilic to clear cytoplasm. The nuclei are round and vesicular with prominent nucleoli. (Hematoxylin and eosin, magnification × 400). D: Immunohistochemical study showed positive immunostaining of tumor cells with CD117. (Immunohistochemistry, magnification × 400).
Dysgerminomas are rare ovarian neoplasms but represent a significant proportion of malignant germ cell tumors, accounting for approximately 33% to 38% of all cases. These tumors predominantly affect individuals under the age of 30, with a median age of diagnosis between 16 and 20 years. In fact, dysgerminomas are the most common malignant ovarian neoplasms in patients under 20 years of age [6,7]. Histologically, dysgerminomas are similar to testicular seminomas as they originate from the primordial germ cells of the embryonic gonads [8]. Dysgerminomas typically manifest with abdominal pain and are often associated with the presence of a pelvic or abdominal mass. However, in some cases, the symptoms may be nonspecific [9]. Dysgerminomas are characterized by a relatively short duration of symptoms, which is often indicative of their rapid tumor growth. While acute pain due to rupture or torsion is uncommon, it may occur in rare cases. Our patient presented with pelvic pain and progressive abdominal swelling, which are typical symptoms associated with dysgerminoma. It is worth noting that dysgerminoma can be associated with conditions such as gonadal dysgenesis and sexual maldevelopment, including Turner syndrome, testicular feminization, and triple X syndrome. In cases of gonadal dysgenesis, dysgerminoma often emerges from a gonadoblastoma. This is most commonly observed in streak gonads but can also occur in intra-abdominal testes [10]. Dysgerminomas often exhibit certain characteristic features and diagnostic markers. Elevated levels of Lactic Dehydrogenase (LDH) are commonly observed in dysgerminoma cases. Additionally, serum levels of Alpha-Fetoprotein (AFP) and beta-human chorionic gonadotropin (beta-HCG) may also be elevated [11]. Ultrasonography is a valuable imaging modality for dysgerminoma detection. Typically, it reveals a well-defined, multi-lobulated, solid heterogeneous mass. Within the mass, hypoechoic fibrous septa are often observed, creating a distinct internal echotexture. Some areas of necrosis or hemorrhage may appear as anechoic regions [12]. Dysgerminomas are well vascularized, and color Doppler imaging typically shows blood flow within the hypoechoic septa. Spectral Doppler analysis commonly exhibits a low-resistance arterial waveform [13]. Accurate surgical staging plays a crucial role in determining the appropriate risk-based treatment for dysgerminomas [12]. In the case of stage IA dysgerminoma, a fertility-preserving surgical approach is usually recommended. This approach involves unilateral salpingo-oophorectomy with staging biopsies while preserving the uterus and contralateral ovary. Adjuvant therapy may not be necessary in such cases, as was the case in the patient under consideration. The primary focus of patient management is to effectively treat the tumor while also preserving fertility, especially in young patients. In cases where dysgerminoma involves both ovaries, bilateral salpingo-oophorectomy and a full staging operation are mandatory. A hysterectomy is only performed if there is tumor involvement in the uterus [1]. Grossly, dysgerminomas typically measure over 10 cm in diameter and have a solid, fleshy tan or white cut surface. They may exhibit areas of necrosis, hemorrhage, or cystic degeneration. Histologically, dysgerminoma presents as sheets or nests of polygonal cells with abundant granular eosinophilic or clear cytoplasm and distinct cell membranes. Cord-like structures, trabeculae, solid tubules, pseudoglands, and a prominent collagenous stroma may also be observed, although less commonly. The tumor cells have medium-sized nuclei with vesicular chromatin, prominent nucleoli, and an elevated mitotic index. Within the tumor, fibrous septa containing lymphocytes (mostly T-cells) and epithelioid histiocytes are often present and can form sarcoid-like granulomas. Syncytiotrophoblastic giant cells are found in about 3% of dysgerminomas. Immunohistochemically, the tumor cells show positive staining for cytoplasmic and membranous PLAP, membranous CD117, and D2-40. They also display diffuse positive nuclear staining for OCT-4, NANOG, and SALL4. EMA is negative, and limited cytoplasmic dot or rim-like staining for cytokeratin may be observed. Syncytiotrophoblastic giant cells show positive staining for hCG. Most dysgerminomas exhibit isochromosome 12p, while 25-50% of tumors have c-kit mutations in exon 17.
Dysgerminomas are considered low-grade malignant neoplasms but can spread if the tumor extends beyond its capsule and involves lymph nodes or blood vessels. Similar to their male counterpart, seminomas, the ovarian dysgerminomas are highly sensitive to radiation and chemotherapy, resulting in an excellent prognosis even after fertility-sparing surgery [14]. Bilateral dysgerminomas are observed in 10% to 15% of cases. The issue of performing a biopsy is still controversial, with some authors suggesting close follow-up to detect any occult dysgerminoma in the remaining ovary. Overall, optimally treated patients with ovarian dysgerminoma have a survival rate exceeding 90%. The stage of the tumor and its size, particularly if it is less than 10 cm, are the most important prognostic factors. Recurrence of Stage 1A dysgerminomas is rare (10% - 15%) and typically occurs within the first two years after diagnosis. However, these recurrences respond well to chemotherapy [15].
In conclusion, dysgerminoma is a rare ovarian malignancy with a favorable prognosis, especially in the early stages. Early diagnosis, accurate staging, and timely surgical intervention are crucial for achieving favorable outcomes. Long-term monitoring and follow-up play a vital role in detecting potential recurrences and ensuring the overall well-being of pediatric patients with dysgerminoma. Collaborative efforts among healthcare professionals are essential to optimize the treatment and long-term prognosis of this rare ovarian malignancy in the pediatric population.
Ethics statement
All procedures performed were in accordance with the ethical standards. The examination was made in accordance with the approved principles.
Written consent from the patient
Published with written consent of the patient.
Detailed author’s contribution
Dr. Faten LIMAIEM and Dr. Khalil SAFFAR prepared, organized, wrote, and edited all aspects of the manuscript. Dr. Faten LIMAIEM performed the gross and microscopic evaluation of the pathology specimen. Dr. Faten LIMAIEM prepared all of the histology figures in the manuscript.
Dr. Ahmed HALOUANI participated in the conception and design of the study, the acquisition of data, analysis, and interpretation of the data.
All authors contributed equally to preparing the manuscript and participated in the final approval of the manuscript before its submission.
- Okunade KS, Yakubu CI, Osuji N. A rare case of ovarian dysgerminoma in a 6-year-old child in Lagos: A case report. Tropical Journal of Obstetrics and Gynaecology. 2017; 34:246-9.
- Biswajit D, Patil CN, Sagar TG. Clinical presentation and outcome of pediatric ovarian germ cell tumor: a study of 40 patients. J Pediatr Hematol Oncol. 2010 Mar;32(2):e54-6. doi: 10.1097/MPH.0b013e3181c5ad9b. PMID: 20168245.
- Otto K, Ebertz O, Matsingou C, Andrikos D, De Wilde RL, Krentel H. Ovarian Dysgerminoma - Challenging Presurgical Diagnosis and Mini-Mally Invasive Treatment. Arch Clin Med Case Rep. 2023;7(1):66-69. doi: 10.26502/acmcr.96550573. Epub 2023 Feb 3. PMID: 36873804; PMCID: PMC9979950.
- Adhikari S, Joti S, Chhetri PK. Paediatric Ovarian Dysgerminoma: A Case Report. JNMA J Nepal Med Assoc. 2022 Nov 2;60(255):985-988. doi: 10.31729/jnma.7894. PMID: 36705173; PMCID: PMC9795100.
- Yoshimura S, Nozaki T, Matsufuji H, Tanio N, Migita M. Metachronous bilateral ovarian tumors: Immature teratoma and dysgerminoma. Pediatr Int. 2022 Jan;64(1):e15251. doi: 10.1111/ped.15251. PMID: 35851512.
- A L Husaini H, Soudy H, El Din Darwish A, Ahmed M, Eltigani A, A L Mubarak M, Sabaa AA, Edesa W, A L-Tweigeri T, Al-Badawi IA. Pure dysgerminoma of the ovary: a single institutional experience of 65 patients. Med Oncol. 2012 Dec;29(4):2944-8. doi: 10.1007/s12032-012-0194-z. Epub 2012 Mar 10. PMID: 22407668.
- Lazebnik N, Balog A, Bennett S, Redline R, Liu J. Ovarian dysgerminoma: a challenging clinical and sonographic diagnosis. J Ultrasound Med. 2009 Oct;28(10):1409-15. doi: 10.7863/jum.2009.28.10.1409. PMID: 19778893.
- Shaaban AM, Rezvani M, Elsayes KM, Baskin H Jr, Mourad A, Foster BR, Jarboe EA, Menias CO. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics. 2014 May-Jun;34(3):777-801. doi: 10.1148/rg.343130067. PMID: 24819795.
- Tatekawa Y, Kemmotsu H, Mouri T, Joe K, Ohkawa H. A case of pediatric ovarian dysgerminoma associated with high serum levels and positive immunohistochemical staining of neuron-specific enolase. J Pediatr Surg. 2004 Sep;39(9):1437-9. doi: 10.1016/j.jpedsurg.2004.05.023. PMID: 15359410.
- Capito C, Arnaud A, Hameury F, Fremond B, Lardy H, Leclair MD, Heloury Y. Dysgerminoma and gonadal dysgenesis: the need for a new diagnosis tree for suspected ovarian tumours. J Pediatr Urol. 2011 Jun;7(3):367-72. doi: 10.1016/j.jpurol.2011.02.021. Epub 2011 Mar 12. PMID: 21402494.
- Kapp DS, Kohorn EI, Merino MJ, LiVolsi VA. Pure dysgerminoma of the ovary with elevated serum human chorionic gonadotropin: diagnostic and therapeutic considerations. Gynecol Oncol. 1985 Feb;20(2):234-44. doi: 10.1016/0090-8258(85)90146-5. PMID: 2579008.
- Varras M, Tsikini A, Polyzos D, Samara Ch, Akrivis Ch. Internal hemorrhage caused by a twisted malignant ovarian dysgerminoma: ultrasonographic findings of a rare case and review of the literature. Clin Exp Obstet Gynecol. 2004;31(1):73-8. PMID: 14998196.
- Kim SH, Kang SB. Ovarian dysgerminoma: color Doppler ultrasonographic findings and comparison with CT and MR imaging findings. J Ultrasound Med. 1995 Nov;14(11):843-8. doi: 10.7863/jum.1995.14.11.843. PMID: 8551550.
- Hosseini B, Leibl M, Stoffman J, Morris A. Two Cases of Hypercalcemia in Pediatric Ovarian Dysgerminoma. J Obstet Gynaecol Can. 2019 May;41(5):660-665. doi: 10.1016/j.jogc.2018.05.004. Epub 2018 Dec 12. PMID: 30551952.
- Guerriero S, Testa AC, Timmerman D, Van Holsbeke C, Ajossa S, Fischerova D, Franchi D, Leone FP, Domali E, Alcazar JL, Parodo G, Mascilini F, Virgilio B, Demidov VN, Lipatenkova J, Valentin L. Imaging of gynecological disease (6): clinical and ultrasound characteristics of ovarian dysgerminoma. Ultrasound Obstet Gynecol. 2011 May;37(5):596-602. doi: 10.1002/uog.8958. Epub 2011 Apr 5. PMID: 21305635.