More Information
Submitted: October 25, 2023 | Approved: January 10, 2024 | Published: January 11, 2024
How to cite this article: Avesani M, Beghini G, Agnoli F, Franchi L, Vianello C. PET TAC and Resting state EEG-fMRI in Evaluation of the Ability to Understand and want in Patients Affected by Dementias with Neuro-psychiatric Disorders and other Mental Disorders. Arch Case Rep. 2024; 8: 001-009.
DOI: 10.29328/journal.acr.1001086
Copyright License: © 2024 Avesani M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: PET TAC; EEG-fMRI; neuropsychiatric disorders; dementias; psychiatric ilnnesses
PET TAC and Resting state EEG-fMRI in Evaluation of the Ability to Understand and want in Patients Affected by Dementias with Neuro-psychiatric Disorders and other Mental Disorders
Mirko Avesani1-6*, Graziella Beghini1, Francesco Agnoli1, Lucilla Franchi2, Camilla Vianello2, Assunta Zamparelli2, Cristiana Trevisan2, Cinzia Scarpa2, Nicola Siliprandi3, Manuela Camicia3, Laura Adami3, Laura Rossi3, Licia Mazzocchi3, Maria Antonietta Conforto3, Lorella Frittoli3, Claudia D’Angelis3, Alfonso Ciccone3, Francesco Paladin3 and Giuseppe Sartori4,6
1University of Verona, Division of Clinical Neurology, Section of Rehabilitative
Neurology, Dementia Center
2AUSL 3 Serenissima, Civil Hospital “SS. Giovanni e Paolo” of Venice, Department of
Medical Sciences, Division of Neurology, Dementias Center
3ASST of Mantua, Civil Hospital “Carlo Poma” of Mantua, Department of Neurological
Sciences, Division of Neurology, Dementias Center
4University of Padua, Degree Course of Law, Department of Criminal Law
5European Forensic Institute (EFI) – Degree Course of Criminology – Section of
Physiological Psychology
6University of Padua, Degree Course of Psychology and Neurological Sciences
*Address for Correspondence: Mirko Avesani, University of Verona, Division of Clinical Neurology, Section of Rehabilitative Neurology, Dementia Center, Italy, Email: [email protected]
Introduction: We strongly believe that RS-fMRI using independent component analysis (ICA) must be considered as a technique to be systematically used in the near future, as positron emission tomography (PET TC) is today. Unfortunately, this technique is not yet used in Italy because, despite the studies just summarized, it is considered “experimental” and not routine without reasonable justification!
Aim of the Study: We present two cases studied with these techniques, after the informed consent obtained by the patients
1) A young woman from Sicily, in whom an RS-fMRI revealed her severe personality disorder, was found capable of insight and strong-willed and was therefore found guilty by the criminal court of the murder of her young son, with a strange motive: RS-fMRI cannot be considered part of the assessment because it is so far considered experimental. PET-TAC was also classified as routine in Italy after a long legal discussion. We hope that all these studies, which are now summarized in this review, will be considered useful, at least in Europe, when a judge has to decide whether to sentence a person with psychological or psychiatric problems or to consider them as a person to be treated in a specific residential home (called REMS in Italy).
2) Another woman from Bergamo, after having killed a neighbor of hers, was, instead, considered not guilty because of her inability to want to kill him, and so admitted to a particular structure (REMS: residence to execution of security measures) to treat her problem, front temporal dementia, with a severe neuropsychiatric disorder (NPS), diagnosed after the crimen was fulfilled.
Conclusions: These two interesting cases demonstrate that in Italy nowadays, we do not have a homogeneous methodology to investigate the ability to understand and want, limiting the study only to personality tests. Here we describe new techniques that may help in this objective.
Neuro-behavioural science is a matter requiring the knowledge of the relationship of cortical structures and, in particular, of a specific neuronal circuitry connected to global brain function. It also requires a knowledge of its perturbations related to the development and progression of neuropathology, because the disruption of these neural networks can be associated with a wide range of neurological and neuropsychiatric disorders, often in neurodegenerative pathologies such as dementias with severe neuropsychiatric disorders [1].
The Intrinsic Connectivity Networks (ICNSs) may be better understood using the technique of studying functional or resting state connectivity and the integration of activity across distant brain regions. In a particular way, it is important to study the Default Mode Network (DMN), which is, nowadays, the most well-characterized among the ICNs [2,3].
The brain’s default mode network
The brain’s default mode network is composed of bilateral and symmetrical cortical areas, located, in mammalians, in the medio/lateral parietal, medial prefrontal, and medio/lateral temporal cortices brains (Figures 1-3) (Table 1). Its discovery was an unexpected consequence of brain-imaging studies first performed with positron emission tomography (cerebral PET TAC). With this useful technique various novel works, now used in parietal activity studies about the ability to understand and want, attention-demanding, and non-self-referential tasks were compared with quiet repose either with eyes closed or with simple visual fixation. The result was very interesting: Default Mode Network (DMN) consistently decreases its activity when compared with activity during these relaxed non-task states [4]. This discovery of the default mode network was the first step aiming to focus on the significance of the intrinsic activity of associative areas in mental health. Presently, studies of the brain’s intrinsic activity, popularly referred to as resting-state studies, have come to play a major role in studies of the human brain in health and disease.
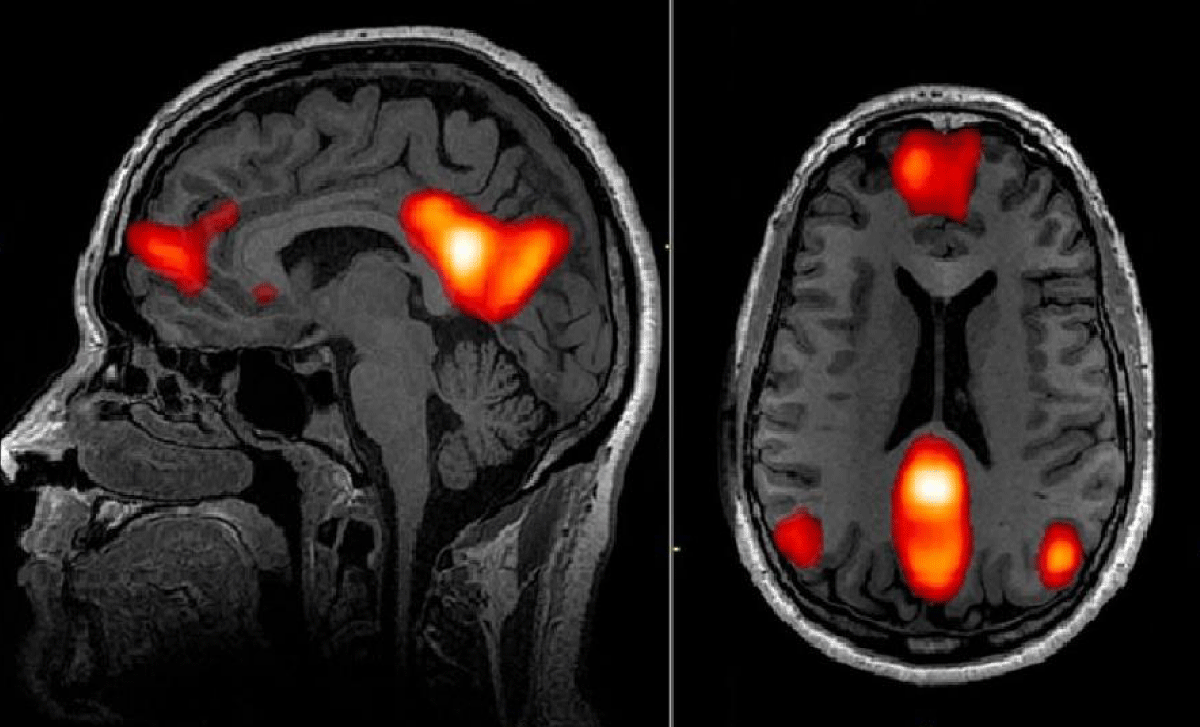
Figure 1: Distribution of Default Mode Network (DMN) in the brain. By Esposito [34]
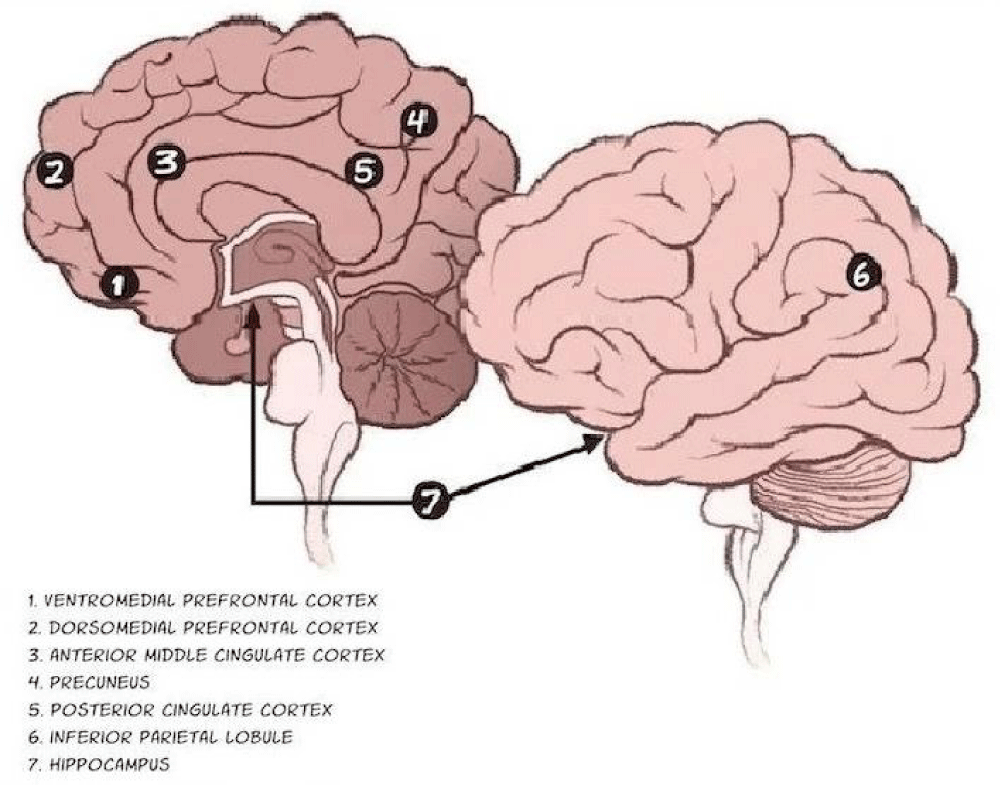
Figure 2: The various parts of Default Mode Network (DMN). Adapted from Raichle [4].
Figure 3: Connectivity of Default Mode Network. In Yellow the main regions of DMN. In red, green, and blue, the connectivity among regions is chromatically codified by the direction of structural crossing (xyz → red-green-blu). By Raichle [3].
| Table 1: Core regions associated with the brain's default network. | ||
| Region | Abrev | Included brain areas |
| Ventral medial prefrontal cortex | VMPFC | 24, 10 m/10 r/10 p, 32ac |
| Posterior cingulate/retosplenial cortex | PCC/Rsp | 29/30, 23/31 |
| Inferior parietal lobule | IPL | 39, 40 |
| Lateral temporal cortext✝ | LTC | 21 |
| Dorsal medial prefrontal cortex | dMPFC | 24, 32ac, lop, 9 |
| Hippocampal formationtt✝✝ | HF+ | Hippocampus proper, EC, PH |
| Notes: Region, abbreviation, end approximate area labels for the core
regions associated with the default network in humans. Lables correspond to those
originally used arodmann for humans with updates by Petrides and Pendya (1994), Vogt, et
al. (1995), Morris, et aI. (2000), and Öngür, et aI. (2003). Labels should considered
approximate because of the uncertain boundaries of the areas end the activation patterns.
✝TLTC is particularly poorly characterized in humans end is therefore the most tentative
estimate. ✝✝TTHF+ includes entorhinal cortex (EC) end surrounding cortex (e.g., parahippocampel cortex; PH). |
||
Activation and deactivation of DMN
The importance of analyzing these regions during parietal activity follows from this neurophysiological evidence: These regions (parts of the DMN) contribute to internally controlled thought processes. Therefore, a mental illness today must also be analyzed with its substrate, i.e. a neurological substrate. Psychiatry and neurology must therefore talk to each other again today as not everything is yet fully understood. In particular, a reduction in task-induced deactivation in the DMN has been associated with increasing age and poorer performance on executive tasks, but the factors underlying these functional changes remain unclear [5].
Currently, we know that the DMN is more active at rest than during the performance of many attention-intensive tasks and is characterized by a high degree of functional connectivity (i.e., correlations between different brain regions).
Why may resting-state EEG-fMRI be useful compared to cerebral PET-TAC to clarify what we do not yet fully understand? It is because functional Magnetic Resonance Imaging (fMRI) studies have shown that the DMN in the healthy brain is associated with stimulus-independent thinking and self-reflection and that greater suppression of the DMN is associated with better performance on attention-demanding tasks [6].
Human cognition is flexible and enables us to select suitable information from memory depending on our current goals. The Multiple Demand cortex (MD), which overlaps with the Frontal-Parietal Control Network (FPCN) and the Dorsal Attention Network (DAN), plays an important role in cognitive flexibility [7]. It shows stronger responses under more demanding conditions in different tasks [8-10] and activation patterns that can adaptively classify task-critical details [11-14].
However, the role of other heteromodal brain regions, such as the regions of the Default Mode Network (DMN), is not well understood [15]. For this reason, co-registration between EEG and resting-state fMRI may help us to better understand this relationship not only in healthy individuals (as a control), but especially in individuals affected by neurodegenerative and mental disorders.
These regions often show reduced activity during attention-intensive tasks, but increase their activity during various forms of complex cognition, many of which are associated with memory or abstract thought. The DMN has been shown to be located within the cortex in regions furthest from those contributing to the sensory and motor systems. Here, several authors [16] consider how their knowledge of the topographic features of the DMN can be used to better understand how this network contributes to cognition and especially (very important in parietal activities) to behaviour. This is because cognition and behaviour are the most important tasks to be studied in people accused of a crime in order to understand their ability to understand and want what they have done, to understand whether they need to pay for the crime or be cured in a specific structure (REMS)
Pathophysiology of DSM
We need to grapple with this important concept: DMN and multiple-demand cortex play opposing roles in cognition, as DMN and multiple-demand regions within the Dorsal Attention Network (DAN) support internal and external cognition, respectively [15]. Thus, while multiple-demand regions may decode current target information, semantically relevant DMN regions could decode conceptual similarity independent of task demands.
Alternatively, DMN regions, such as the cortex with multiple demands, show sensitivity to changing task demands, as both networks dynamically change their connectivity patterns depending on the context. With this knowledge, we were able to decode semantic categories and current target information using whole-brain searchlight decoding. As expected, the multiple-demand cortex, including the DAN and frontoparietal control network, represented information about topically relevant conceptual features. Similar decoding results were found in the DMN, including the angular gyrus and posterior cingulate cortex, suggesting that the DMN and multiple demand regions may support the same function and are not strictly competing. The semantic category could be decoded in the lateral occipital cortex independently of task demands, but not in most regions of the DMN. Conceptual information related to the current target dominates the multivariate response within the DMN, which supports flexible retrieval by adapting its response to the demands of the task, as did regions of the multiple-demand cortex. Wang used multivoxel pattern analysis to test contrasting explanations for the function of the Default Mode Network (DMN) [15]. According to one view, semantically relevant parts of the DMN represent conceptual similarity, regardless of the task context. In another view, the DMN tracks the changing requirements of the task. In this semantic feature mapping task, participants had to link conceptual knowledge to the goals of the task, so that successive choices were based on different features of the items. So the important concept we now know is this: DMN regions are associated with cognitive control, and so full knowledge of these regions can explain how the DMN supports flexible cognition [15]. Flexible cognition is often altered in mental illness and in neurodegenerative dementias with neuropsychiatric disorders.
The DMN and DAN are thought to be responsible for inward and outward cognition, respectively, and are functionally connected to different subsystems of the FPCN [17-19]. The DMN is highly heteromodal and is thought to support information integration [20,21], which is relevant both for long-term episodic memory [22] and for semantic cognition [23-25]. Semantically relevant DMN regions, including the left Angular Gyrus (AG) and lateral temporal cortex, show less deactivation compared to rest when semantic and non-semantic tasks are compared [26-28], even when task difficulty is taken into account [29-31]. These observations suggest that DMN might support similarity structures in long-term memory, such as global conceptual similarity [32,33], as well as goal information when this information is retrieved from memory.
Recent data on DSM in brain organization
Actually what we know about brain organization is that neural function is organized along a connectivity gradient from unimodal regions of the sensorimotor cortex, through executive regions to a transmodal default mode network.
It has been investigated on whether this graded view of neural organization can help explain how decision-making changes in situations that differ in their consistency with long-term knowledge. It was found that the brain’s response to the task gradient varied systematically along the connectivity gradient, with the strongest response occurring in the default-mode network when the test and target objects conceptually overlapped strongly. This graded functional change was observed in multiple brain regions and within individual brains and could not be readily explained by the difficulty of the task.
In addition, the gradient captured the spatial arrangement of the networks involved in semantic processing, providing an organizing principle for controlled semantic cognition throughout the cortex. In this way, the cortex is organized to support semantic decision-making in both highly familiar and less familiar situations [33].
DSM in Neuropsychiatric disorders, a cause of crime
Neuropsychiatric disorders are associated with abnormal function of the default mode network (DMN). Imaging studies using functional magnetic resonance imaging (fMRI) have shown that the DMN in the healthy brain is associated with stimulus-independent thinking and self-reflection and that greater suppression of the DMN is associated with better performance on tasks requiring attention. In schizophrenia and depression, the DMN is often hyperactivated and hyperconnected. In schizophrenia, this may be related to overly intense self-reference and impaired attention and working memory. In depression, DMN hyperactivity may be related to negative rumination. These findings are considered in the context of what is known about psychological functions supported by the DMN and changes in the DMN in other neuropsychiatric disorders [6].
DMN alteration in Dementia
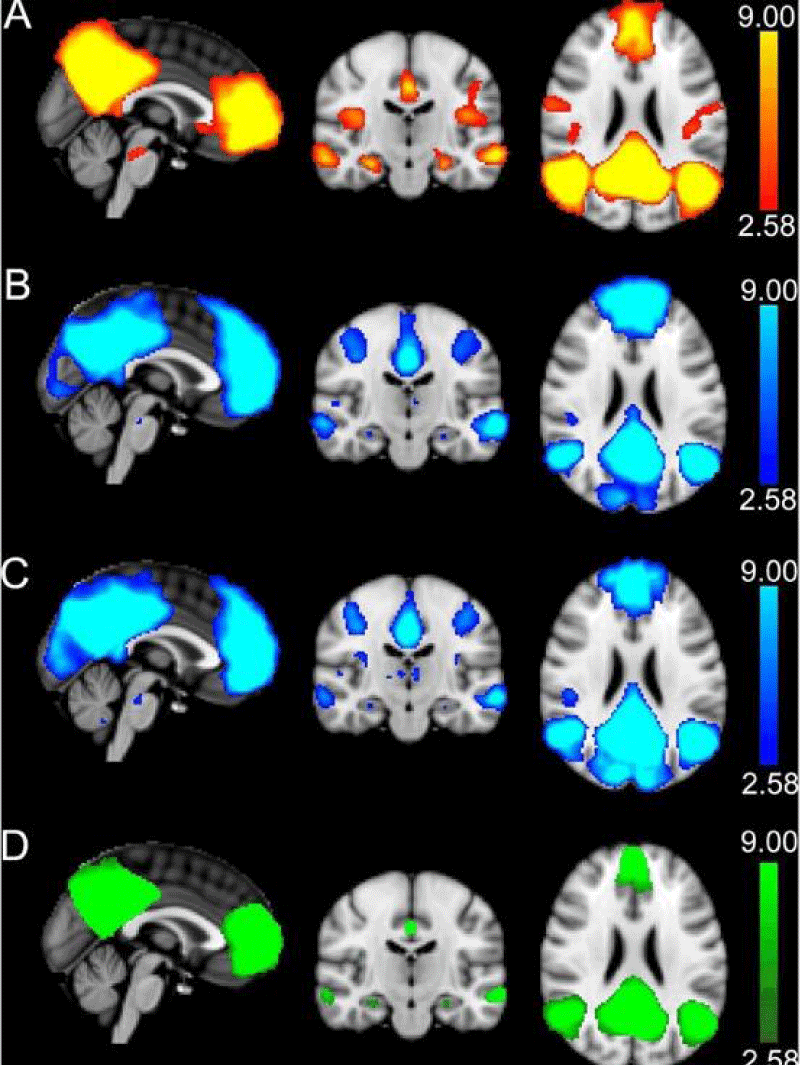
The contribution of white matter (WM) microstructure, WM hyperintensities, and Alzheimer’s disease pathology to age-related changes in DMN function has also been investigated [5]. Thirty-five cognitively normal older adults and 29 younger adults underwent fMRI imaging of working memory and diffusion tensor imaging. In the older adults, tau and Aβ42 were measured in CSF (markers of AD pathology) and WMH in FLAIR imaging (marker of cerebrovascular disease). A number of regions showing deactivation of the DMN were identified, as well as a number of interconnected WM pathways (DMN-WM) common to both age groups. There were negative associations between DMN deactivation and task performance in older adults, consistent with previous studies. Reduced DMN deactivation was associated with AD pathology and WM microstructure, but not WMH volume. Mediation analyses showed that WM microstructure mediates the decrease in DMN deactivation associated with both ageing and AD pathology. These results suggest that AD pathology may exert a “second hit” on WM microstructure, in addition to the effects of age, both of which contribute to decreased DMN deactivation in older adults. Figures 4,5 can help us understand this change.
Figure 4: Identification of DMN with the results of independent component analysis (ICA) in Alzheimer’s disease. A: ICA was used to identify the DMN components in RS-fMRI data. B-C: ICA was performed separately for run 1 (B) and run 2 (C) of task-based fMRI. The task components were averaged and masked by the RS-fMRI component to form a final mask of the DMN (C). The clusters in this final mask were used for all functional analyses and as seeds for probabilistic tractography. A-D: The DMN regions overlap with the MNI151 T1 2mm3 standard brain. The colour scale is shown on the right, with the values representing the minimum and the maximum Z-values. By Brown, et al. [5],
Figure 5: Comparison of the decreased functional connectivity in Alzheimer’s disease to controls (healthy subjects). By Whitfield-Gabrieli [6].
THE NEW TECHNIQUES USEFUL TO INTEGRATE THE CLASSIC NEUROPSYCHOLOGICAL EXAMINATION IN PEOPLE ACCUSED OF A CRIME AND TO BE STUDIED IN ORDER THEIR WILL OF UNDERSTAND AND WANT
Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI
Resting-state functional Magnetic Resonance Imaging (RS-fMRI), combined with EEG recording (EEG-fMRI coregistration) in continuous (and not triggered) modality, is a technique useful to study the spontaneous correlations of Blood-Oxygen-Level-Dependent (BOLD) signals across different brain regions, associated with the spectrum of strong different brain activities (alpha, beta, gamma, theta, delta waves). With these functional connectivity tools (fMRI in resting state, or coregistration EEG-fMRI in resting state), it is possible to study a specific RS-fMRI network, called the “Default-Mode” (DM) network, which includes cortical regions that are deactivated in fMRI experiments with cognitive tasks. Previous works have reported a significant effect of aging on DM regions’ activity. Independent Component Analysis (ICA) is often used for generating spatially distributed DM functional connectivity patterns from RS-fMRI data without the need for a reference region [34].
This aspect and the relatively easy setup of an RS-fMRI experiment even in clinical trials have boosted the combined use of RS-fMRI and ICA-based DM analysis for noninvasive research of brain disorders. A study [34] considered different strategies for combining ICA results from individual-level and population-level analyses and used them to evaluate and predict the effect of aging on the DM component. RS-fMRI data from 20 healthy subjects and a previously developed group-level ICA methodology were used to generate group DM maps, and it was shown that the overall ICA-DM connectivity was negatively correlated with age. A negative correlation of the ICA voxel weights with age existed in all DM regions at a variable degree. So it generated a distributed DM spatial template and evaluated the correlation of each individual DM component fit to this template with age.
Abnormalities in functional connectivity in borderline personality (BP) disorder: Correlations with metacognition and emotion dysregulation.
In the literature, we found the first studies reporting functional abnormalities at rest in Borderline Personality Disorder (BPD), but their relationship with the clinical aspect of the illness is, currently still unclear.
In particular, a study [35] aimed to assess Functional Connectivity (FC) in BPD patients and its association with BPD clinical features. BPD patients and Healthy Controls (HC) underwent a multidimensional assessment and resting-state fMRI. Independent Component Analysis (ICA) was performed to identify three resting-state networks: Default Mode Network (DMN), Salience Network (SN), and Executive Control Network (ECN). FC differences between BPD and HC were assessed with voxel-wise two-sample t-tests. Additionally, the study investigated the mean FC within each network and the relationship between connectivity measures and BPD clinical features. Patients showed significantly lower mean FC in the DMN and SN, while, at the local level, a cluster of lower functional connectivity emerged in the posterior cingulate cortex of the DMN.
The DMN connectivity was positively correlated with the anger-state intensity and expression, while the SN connectivity was positively correlated with metacognitive abilities and a negative correlation emerged with interpersonal aggression. The conclusion of this interesting study is that dysfunctional connectivity within these networks might explain the clinical features of BPD patients. Another reason to consider the usefulness of this technique in parietal studies on people with BPS accused of a crime. At now, this technique is ignored, and so may cause several condemnations in the place of admissions to particular structures of cure (REMS).
Another study [36] analyzed network analysis of functional brain connectivity in borderline personality disorder using the same technique, resting-state fMRI with ICA, confirming its usefulness in parietal activities. The study was organized because little is known about the topological organizations of brain networks in BPD.
In fact, also in this study, neuroimaging research on BPD has revealed structural and functional abnormalities in specific brain regions and connections. The study collected resting-state functional Magnetic Resonance Imaging (fMRI) data from 20 patients with BPD and 10 Healthy Controls (HC) and constructed frequency-specific functional brain networks by correlating wavelet-filtered fMRI signals from 82 cortical and sub-cortical regions. A complex network analysis was used to investigate the topological properties of the brain networks, and a network-based statistic was used to identify functional disconnections in patients. In the 0.03 - 0.06 Hz frequency band, compared to controls, patients with BPD showed significantly larger measures of global network topology, including the size of the largest connected graph component, clustering coefficient, small-worldness, and local efficiency, indicating increased local cliquishness of the functional brain network. Compared to controls, patients showed lower nodal centrality at several hub nodes but greater centrality at several non-hub nodes in the network. Furthermore, an interconnected subnetwork in the 0.03-0.06 Hz frequency band was identified that showed significantly lower connectivity in patients. The links in the subnetwork were mainly long-distance connections between regions located at different lobes, and the mean connectivity of this subnetwork was negatively correlated with the increased global topology measures. Lastly, the key network measures showed high correlations with several clinical symptom scores and classified BPD patients against healthy controls with high accuracy based on linear discriminant analysis. The abnormal topological properties and connectivity found in this study may add new knowledge to the current understanding of functional brain networks in BPD. However, due to the limitation of small sample sizes, the results of the current study should be viewed as exploratory and need to be validated on large samples in future works.
Disrupted intrinsic functional brain topology in patients with major depressive disorder
Aberrant topological organization of whole-brain networks has been reported in an interesting study [37] of patients with Major Depressive Disorder (MDD).
To address this issue, a big data sample of MDD patients from the REST-meta-MDD Project, including 821 MDD patients and 765 Normal Controls (NCs) from 16 sites, was used.
The whole-brain functional networks were analyzed and topological features (global and local efficiency, nodal efficiency, and degree) using graph theory-based methods were extracted.
The results found decreased global and local efficiency in patients with MDD compared to NCs. Patients with MDD were characterized by decreased nodal degrees in the Somatomotor Network (SMN), dorsal attention network (DAN), and Visual Network (VN) and decreased nodal efficiency in the Default Mode Network (DMN), SMN, DAN, and VN. These topological differences were mostly driven by recurrent MDD patients, rather than First-Episode Drug-Naive (FEDN) patients with MDD. In this highly powered multisite study, a disrupted topological architecture of functional brain networks in MDD was evident, suggesting both locally and globally decreased efficiency in brain networks.
Future approaches for people accused of a crime and to study about their will to understand and want
We are strongly convinced that RS-fMRI, using ICA, must be considered, in a next future, a technique to be systematically used as now is used Positron Emission TAC (PET TC). Currently, in Italy, this technique is unfortunately not always used because regardless of the studies just enumerated, it is still considered “experimental” and not routine, in the absence of a reasonable rationale.
We have faced two different cases with different decisions by Penal Courts. The patients were studied after they signed an informed consent.
First case: In the first case, a young woman from Sicily, with a RS-fMRI clear about her severe personality disorder (BP), was considered able to understand and want and so considered guilty by the Penal Court for the murder of her little son, with a strange motive: RS-fMRI could not be considered as part of evaluation, because it is still experimental. But also her PET TAC was in line with the conclusion of RS-fMRI. Penal Court decided to revise her decision when “RS-fMRI will become a routine technique”.
It is to be noted that PET-TAC, in Italy, was initially considered only experimental. It was considered routine after a long juridical discussion, during the criminal trial for the murder of Italian stylist Gucci (Reggiani’s criminal trial, for the name of her wife, who organized the crime). Since that criminal trial, PET-TAC has routinely been used during parietal activities to detect the ability to understand and wants of the person responsible for the crime.
We hope that all these studies, now summarized in this review, will be useful, at least in Europe, when a judge must decide if to sentence or to consider a subject to be addressed in a particular residence (in Italy defined REMS) of people affected by psychological or psychiatric problems.
Second case: A positive partial opening towards these new techniques was observed in the second case when the Penal Court of Bergamo was called to judge a woman who committed a never-studied-before crime, responsible for having killed a neighbor with four gunshots.
She was always a correct woman, never known in the penal field. But, in the months previous to the crime, she started to manifest in herself a changed character, with deficits in working memory, organization, planning, and instrumental daily life autonomies (IADL) but not in daily life autonomies (ADL). At her home, she kept in a drawer, a pistol of her husband, deceased 10 years before.
Her sons never could have imagined that a woman so kind and mild, devoted only to the memory of her husband (her daily voyage was to church for Holy Mass and, then, to the graveyard to visit the husband’s tomb), could so quickly change her character and they considered the situation only as deriving from the stress of living alone.
But she became unable to use her money, to keep the house, to prepare the meals, and to take her usual drugs, and, with a major susceptibility to provocations.
These signs, after the crime, were analyzed in a REMS to which she was allocated (Castiglione delle Stiviere), waiting for a criminal trial. So during this period a Judge (in Italy defined GIP: The Judge for preliminary investigations) hypothesized the possibility of a mental illness, and decided to make the patient be evaluated by the Memory and Dementia Clinic inside Neurology Division of Civil Hospital of Mantua. This decision led to the diagnosis of front temporal dementia, a frontal behavioural variant, as a cause of all these signs.
So, the patient was considered unable to want and so she was not condemned as responsible for a murder (that is guilty), but she was confirmed as needing to be treated in a particular residence to facilitate social gatherings.
We aim to start, in a brief time, work on every subject with the clinical suspect of a psychiatric or psychological disorder when the judge demands him/her an evaluation
In conclusion, this second case, let us anticipate a new approach by the penal Court, both in Italy and in Europe, towards mental illness as the cause of a crime. We can expect this evolution because several studies have demonstrated the alteration of DMN in Cognitive and psychiatric disorders.
- Mohan A, Roberto AJ, Mohan A, Lorenzo A, Jones K, Carney MJ, Liogier-Weyback L, Hwang S, Lapidus KA. The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale J Biol Med. 2016 Mar 24;89(1):49-57. PMID: 27505016; PMCID: PMC4797836.
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008 Mar;1124:1-38. doi: 10.1196/annals.1440.011. PMID: 18400922.
- Raichle ME. A default mode of brain function. Proc Natl Acad Sci USA. 2001; 98(2):676–682. doi: 10.1073/pnas.98.2.676.
- Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015 Jul 8;38:433-47. doi: 10.1146/annurev-neuro-071013-014030. Epub 2015 May 4. PMID: 25938726.
- Brown CA. Age and Alzheimer's pathology disrupt default mode network functioning via alterations in white matter microstructure but not hyperintensities. Cortex. 2018; 104: 58–74. doi: 10.1016/j.cortex.2018.04.006.
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi: 10.1146/annurev-clinpsy-032511-143049. Epub 2012 Jan 6. PMID: 22224834.
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010; 14:172–179. doi: 10.1016/j.tics.2010.01.004.
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16428-33. doi: 10.1073/pnas.1112937108. Epub 2011 Sep 1. PMID: 21885736; PMCID: PMC3182706.
- Turnbull A, Wang HT, Schooler JW, Jefferies E, Margulies DS, Smallwood J. The ebb and flow of attention: Between-subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. Neuroimage. 2019 Jan 15;185:286-299. doi: 10.1016/j.neuroimage.2018.09.069. Epub 2018 Sep 25. PMID: 30266263.
- Turnbull A. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat Commun. 2019; 10:3816. doi: 10.1038/s41467-019-11764-y.
- Erez Y. Discrimination of visual categories based on behavioral relevance in widespread regions of frontoparietal cortex. J Neurosci. 2015; 35:12383–12393. doi:10.1523/JNEUROSCI.1134-15.2015
- Cole MW, Ito T, Braver TS. The Behavioral Relevance of Task Information in Human Prefrontal Cortex. Cereb Cortex. 2016 Jun;26(6):2497-505. doi: 10.1093/cercor/bhv072. Epub 2015 Apr 13. PMID: 25870233; PMCID: PMC4869805.
- Bracci S, Daniels N, Op de Beeck H. Task Context Overrules Object- and Category-Related Representational Content in the Human Parietal Cortex. Cereb Cortex. 2017 Jan 1;27(1):310-321. doi: 10.1093/cercor/bhw419. PMID: 28108492; PMCID: PMC5939221.
- Qiao L. Dynamic trial-by-trial recoding of task-set representations in the frontoparietal cortex mediates behavioral flexibility. J Neurosci. 2017; 37:11037–11050. doi: 10.1523/JNEUROSCI.0935-17.2017
- Wang X, Gao Z, Smallwood J, Jefferies E. Both Default and Multiple-Demand Regions Represent Semantic Goal Information. J Neurosci. 2021 Apr 21;41(16):3679-3691. doi: 10.1523/JNEUROSCI.1782-20.2021. Epub 2021 Mar 4. PMID: 33664130; PMCID: PMC8055078.
- Smallwood J, Bernhardt BC, Leech R, Bzdok D, Jefferies E, Margulies DS. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci. 2021 Aug;22(8):503-513. doi: 10.1038/s41583-021-00474-4. Epub 2021 Jul 5. PMID: 34226715.
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010 Oct 15;53(1):303-17. doi: 10.1016/j.neuroimage.2010.06.016. Epub 2010 Jun 18. PMID: 20600998; PMCID: PMC2914129.
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013 Jan;25(1):74-86. doi: 10.1162/jocn_a_00281. Epub 2012 Aug 20. PMID: 22905821; PMCID: PMC3816715.
- Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, Christoff K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1598-E1607. doi: 10.1073/pnas.1715766115. Epub 2018 Jan 30. Erratum in: Proc Natl Acad Sci U S A. 2018 Mar 12;: PMID: 29382744; PMCID: PMC5816169.
- Simony E, Honey CJ, Chen J, Lositsky O, Yeshurun Y, Wiesel A, Hasson U. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat Commun. 2016 Jul 18;7:12141. doi: 10.1038/ncomms12141. PMID: 27424918; PMCID: PMC4960303.
- Lanzoni L, Ravasio D, Thompson H, Vatansever D, Margulies D, Smallwood J, Jefferies E. The role of default mode network in semantic cue integration. Neuroimage. 2020 Oct 1;219:117019. doi: 10.1016/j.neuroimage.2020.117019. Epub 2020 Jun 6. PMID: 32522664; PMCID: PMC7443705.
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011 Mar 23;31(12):4407-20. doi: 10.1523/JNEUROSCI.3335-10.2011. PMID: 21430142; PMCID: PMC3098040.
- Binder JR. The neurobiology of semantic memory. Trends Cogn Sci. 2011; 15:527–536. doi: 10.1016/j.tics.2011.10.001
- Wirth M. Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. Neuroimage. 2011; 54:3057–3066. doi: 10.1016/j.neuroimage.2010.10.039.
- Krieger-Redwood K, Jefferies E, Karapanagiotidis T, Seymour R, Nunes A, Ang JWA, Majernikova V, Mollo G, Smallwood J. Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. Neuroimage. 2016 Nov 1;141:366-377. doi: 10.1016/j.neuroimage.2016.07.060. Epub 2016 Jul 30. PMID: 27485753; PMCID: PMC5035136.
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999 Jan;11(1):80-95. doi: 10.1162/089892999563265. PMID: 9950716.
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009 Dec;19(12):2767-96. doi: 10.1093/cercor/bhp055. Epub 2009 Mar 27. PMID: 19329570; PMCID: PMC2774390.
- Humphreys GF, Hoffman P, Visser M, Binney RJ, Lambon Ralph MA. Establishing task- and modality-dependent dissociations between the semantic and default mode networks. Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7857-62. doi: 10.1073/pnas.1422760112. Epub 2015 Jun 8. PMID: 26056304; PMCID: PMC4485123.
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci. 2005 Jun;17(6):905-17. doi: 10.1162/0898929054021102. PMID: 16021798.
- Seghier ML, Fagan E, Price CJ. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci. 2010 Dec 15;30(50):16809-17. doi: 10.1523/JNEUROSCI.3377-10.2010. PMID: 21159952; PMCID: PMC3105816.
- Murphy C. Distant from input: evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage. 2018; 171:393–401. doi: 10.1016/j.neuroimage.2018.01.017.
- Murphy C. Fractionating the anterior temporal lobe: MVPA reveals differential responses to input and conceptual modality. Neuroimage. 2017. doi: 10.1016/j.neuroimage.2016.11.067.
- Wang X, Margulies DS, Smallwood J, Jefferies E. A gradient from long-term memory to novel cognition: Transitions through default mode and executive cortex. Neuroimage. 2020 Oct 15;220:117074. doi: 10.1016/j.neuroimage.2020.117074. Epub 2020 Jun 20. PMID: 32574804; PMCID: PMC7573535.
- Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Goebel R, Di Salle F. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imaging. 2008 Sep;26(7):905-13. doi: 10.1016/j.mri.2008.01.045. Epub 2008 May 16. PMID: 18486388.
- Quattrini G, Pini L, Pievani M, Magni LR, Lanfredi M, Ferrari C, Boccardi M, Bignotti S, Magnaldi S, Cobelli M, Rillosi L, Beneduce R, Rossi G, Frisoni GB, Rossi R. Abnormalities in functional connectivity in borderline personality disorder: Correlations with metacognition and emotion dysregulation. Psychiatry Res Neuroimaging. 2019 Jan 30;283:118-124. doi: 10.1016/j.pscychresns.2018.12.010. Epub 2018 Dec 20. PMID: 30591402.
- Xu T, Cullen KR, Mueller B, Schreiner MW, Lim KO, Schulz SC, Parhi KK. Network analysis of functional brain connectivity in borderline personality disorder using resting-state fMRI. Neuroimage Clin. 2016 Feb 18;11:302-315. doi: 10.1016/j.nicl.2016.02.006. PMID: 26977400; PMCID: PMC4782004.
- Yang H, Chen X, Chen ZB, Li L, Li XY, Castellanos FX, Bai TJ, Bo QJ, Cao J, Chang ZK, Chen GM, Chen NX, Chen W, Cheng C, Cheng YQ, Cui XL, Duan J, Fang Y, Gong QY, Guo WB, Hou ZH, Hu L, Kuang L, Li F, et al. Disrupted intrinsic functional brain topology in patients with major depressive disorder. Mol Psychiatry. 2021 Dec;26(12):7363-7371. doi: 10.1038/s41380-021-01247-2. Epub 2021 Aug 12. PMID: 34385597; PMCID: PMC8873016.