More Information
Submitted: December 11, 2023 | Approved: December 27, 2023 | Published: December 28, 2023
How to cite this article: Singh N, Materum P, Zavala A, Sheth R, Kapenhas E. Patient with Fibroadenoma on Biopsy, Found to have Phyllodes on Final Pathology: A Case Report. Arch Case Rep. 2023; 7: 083-086.
DOI: 10.29328/journal.acr.1001085
Copyright License: © 2023 Singh N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Phyllodes tumor; Fibroadenomas; Breast surgery; Surgical excision
Patient with Fibroadenoma on Biopsy, Found to have Phyllodes on Final Pathology: A Case Presentation
Niharika Singh1* , Pons Materum2, Artemio Zavala2, Riddhish
Sheth2 and Edna Kapenhas3
, Pons Materum2, Artemio Zavala2, Riddhish
Sheth2 and Edna Kapenhas3
1Department of Surgery, Stony Brook University Hospital, Stony Brook, NY 11794,
USA
2Department of Pathology, Stony Brook University Hospital, Stony Brook, NY 11794,
USA
3Department of Breast Surgery, Stony Brook Southampton Hospital, Southampton, NY
11968, USA
*Address for Correspondence: Dr. Niharika Singh, Department of Surgery, Stony Brook University Hospital Stony Brook, NY 11794, USA, Email: [email protected]
Distinguishing between fibroadenomas and phyllodes tumors is a challenge in breast surgery, despite advances in both radiology and pathology. In this case report, we analyze a patient presenting with a breast mass with multiple core needle biopsy results consistent with fibroadenoma, who underwent enucleation and was found to have phyllodes tumor on final pathology, thereby requiring surgical re-excision. This case report highlights the importance of patient clinical presentation in differentiating fibroadenomas and phyllodes tumors and explores how to achieve appropriate margins upon surgical re-excision after prior enucleation of phyllodes tumor via ultrasound localization of a seroma.
The distinction between fibroadenomas and phyllodes tumors continues to be a diagnostic dilemma. The management of these lesions is different. Fibroadenomas can be safely observed or an enucleation can be performed. In contrast, it is recommended that phyllodes tumors be excised with 1 cm margins to prevent recurrence.
Fibroadenomas and phyllodes tumors are both breast fibroepithelial lesions [1]. Phyllodes tumors are classified into benign, borderline, and malignant grades based on stromal cellularity and atypia, mitotic count, and degree of overgrowth [2]. Benign phyllodes tumor has overlapping features with cellular fibroadenoma, whereas malignant phyllodes tumors can be confused with primary breast sarcoma [2]. Some suggest that a subset of phyllodes tumor progress from fibroadenoma [1,3].
Clinical features are used to help distinguish between fibroadenomas and phyllodes tumors. One study showed that phyllodes tumors were statistically more likely to present with symptoms of breast pain [4]. Another study found that the average age of presentation for phyllodes tumors was older than those with fibroadenoma (39 years vs. 23 years), and phyllodes tumors were more likely to be larger than fibroadenomas at surgical excision (5 cm vs. 2 cm) [5].
On imaging, phyllodes tumors are statistically more likely to have clefts or round cysts on ultrasound or have a core needle biopsy specimen consistent with phyllodes tumor [4]. On MRI, findings of tumor size greater than or equal to 3 cm, irregularity, lobulated margins (100% vs. 90%), internal cystic areas (46% vs. 27%), and hypervascularity were statistically more likely to be associated with phyllodes tumors than fibroadenomas [6,7]. Distinguishing fibroadenomas and phyllodes continues to be a question of clinical interest, even artificial intelligence has been suggested as a means of distinguishing ultrasound images of phyllodes tumors versus fibroadenoma [8,9].
On cytology, phyllodes tumors were more likely to have folded epithelial sheets, stromal fragments, spindle stromal cell nuclei, and cellular atypia than fibroadenomas on fine needle aspiration [5]. There are no established criteria for evaluation of immunochemistry stains (such as Ki-67, p53, and beta-catenin) to help stratify fibroadenoma and phyllodes tumors [10]. Excisional biopsies remain the gold standard for differentiating fibroadenomas and phyllodes tumors; however, core needle biopsies are less invasive and may also provide important diagnostic information. In a study analyzing core needle biopsies that raised the possibility of phyllodes tumors, it was determined that the negative predicative value of core needle biopsies for phyllodes tumors was 93% and the positive predicative value was 83% [11].
As phyllodes tumors can present similarly to fibroadenomas, they can be inappropriately enucleated. It is recommended that if a 1 cm margin has not been obtained, the patient should undergo re-excision to obtain appropriate margins for phyllodes [12]. Mangi, et al. found that post-excision recurrences occurred in cases with positive margins or margins of < 1 cm, with recurrence rates of 15% - 20% being cited [12].
In this case report, we analyze a patient presenting with a breast mass with multiple core needle biopsy results consistent with fibroadenoma, who underwent enucleation and was found to have phyllodes tumor on final pathology, thereby requiring surgical re-excision.
A 43-year-old female with a past medical history of anxiety presents with a palpable left breast mass. Her family history is remarkable for breast cancer in her mother and maternal aunt, but her mother’s genetic testing was negative. The patient had menarche at 16 years old, did not have any biological children, and had used oral contraceptive pills for 13 years. Her Gail Model showed a 3.2% 5-year risk of breast cancer and 26% lifetime risk of breast cancer. Tyrer-Cuzick Risk Assessment indicated a 4.4% 10-year risk and 24% lifetime risk of breast cancer.
In 2016, the patient initially presented for her left breast mass. On sonogram, the mass was 2.1 cm, BIRADS 4. The patient then underwent an ultrasound-guided core biopsy, which was consistent with a fibroadenoma.
In 2019, the patient re-presented with a subjectively enlarging mass. A sonogram showed a 4.5 cm complex cystic mass in the upper outer quadrant of her left breast, BIRADS 4B. An ultrasound-guided core biopsy was performed that was consistent with a fibroadenoma. At this point, it was recommended that the patient undergo an excision of the mass. She scheduled the surgery but subsequently canceled the surgery due to the COVID-19 pandemic.
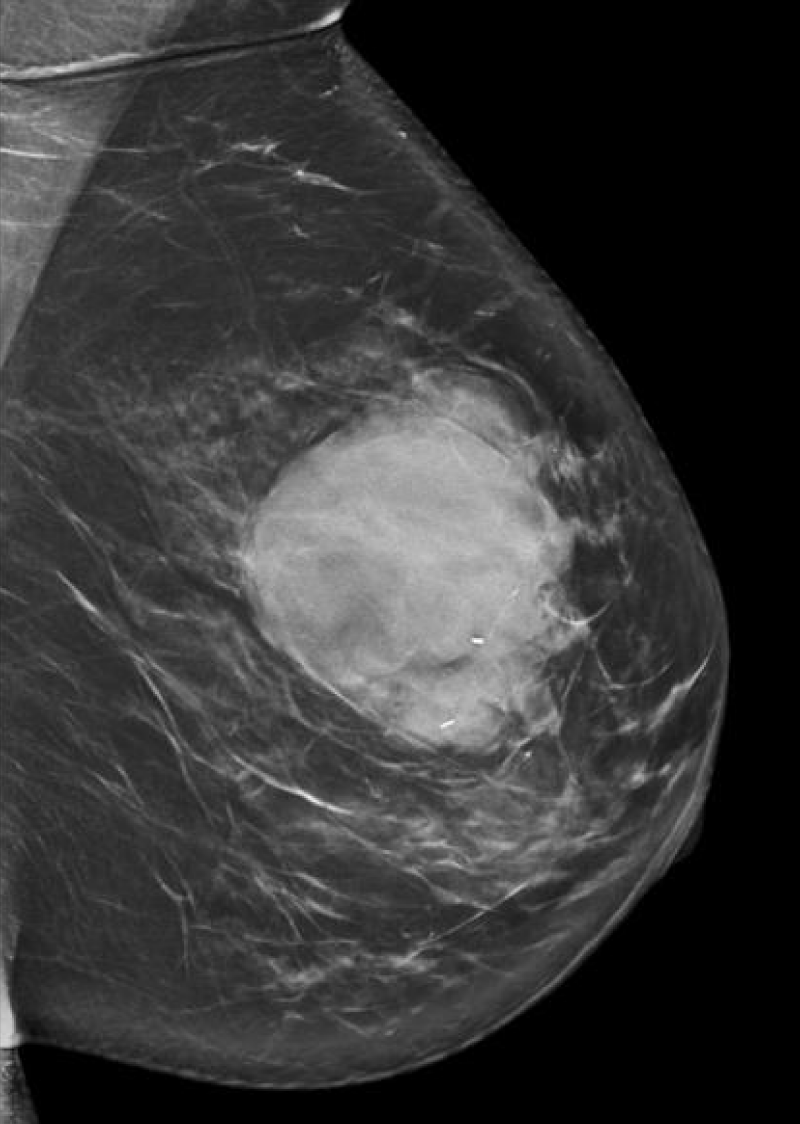
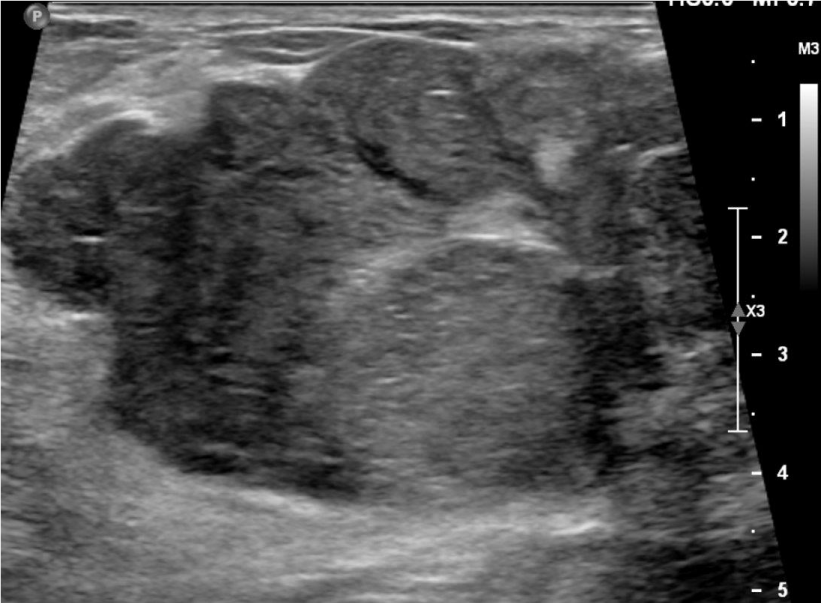
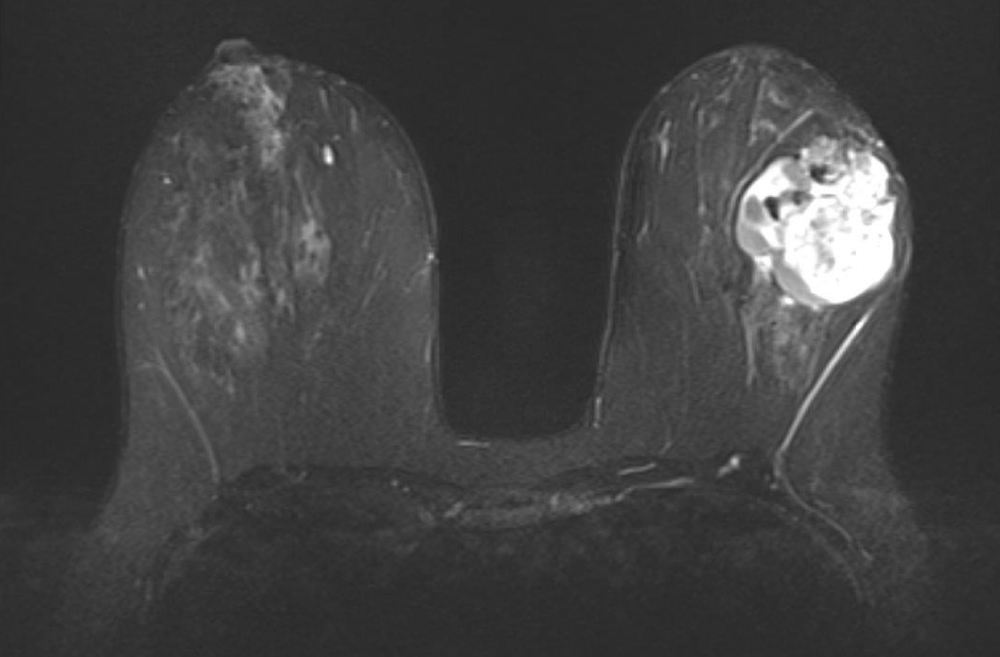
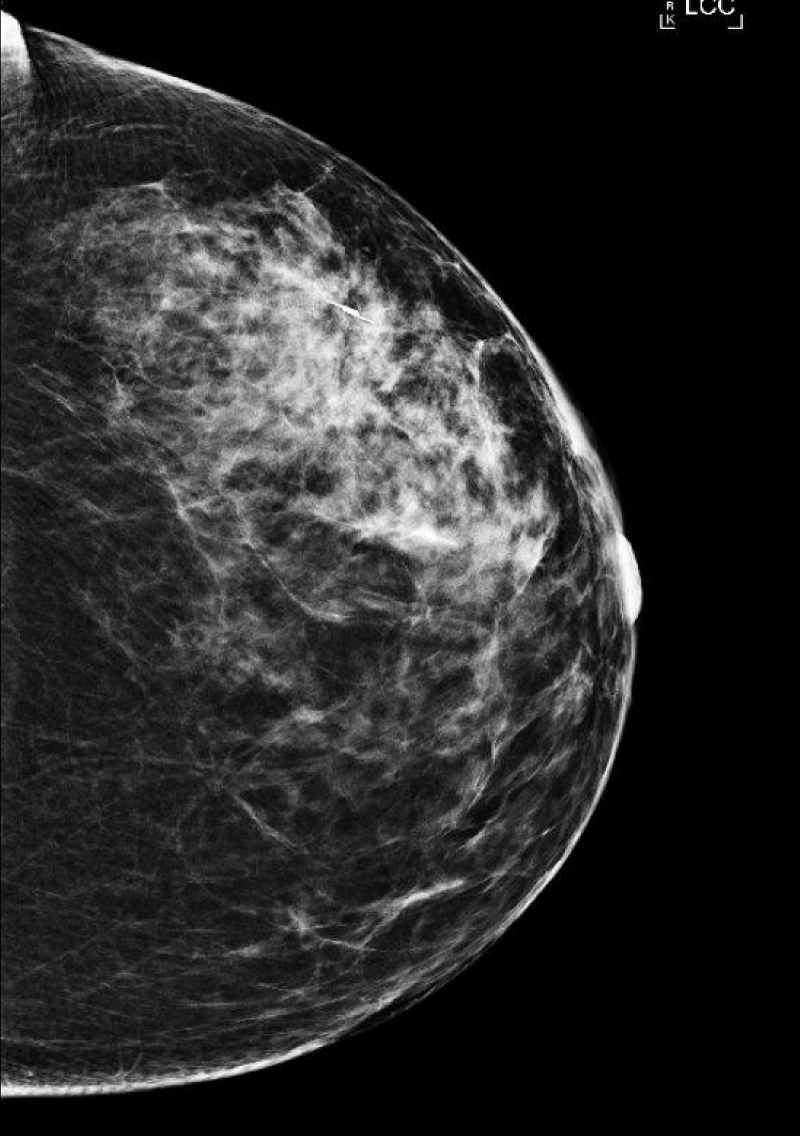
She next re-presented in 2023 due to the increased size of the mass. On mammogram, the mass measured 6 cm x 6 cm (Figure 1), and on ultrasound, the patient’s mass was 7.1 x 4.0 cm (Figure 2). MRI showed a 6.3 cm x 3.5 cm x 5.7 cm mass with more heterogenous enhancement, BIRADS 4 (Figure 3). She underwent an excisional biopsy which revealed a lobulated mass 8.6 cm x 6.4 cm with bluish cystic areas and brown cystic fluid. Cytology of the fluid showed atypical ductal cells. On histology, the tumor exhibited mild to moderate stromal hypercellularity with sparse mitoses and modest cellular atypia without overt stromal overgrowth.
Figure 1: Mammogram of patient in 2023, showing 6 cm x 6 cm left breast mass.
Figure 2: Ultrasound of patient in 2023, showing a 71 x 40 mm mass.
Figure 3: MRI revealed a 6.3 cm x 3.5 cm x 5.7 cm mass with more heterogenous enhancement.
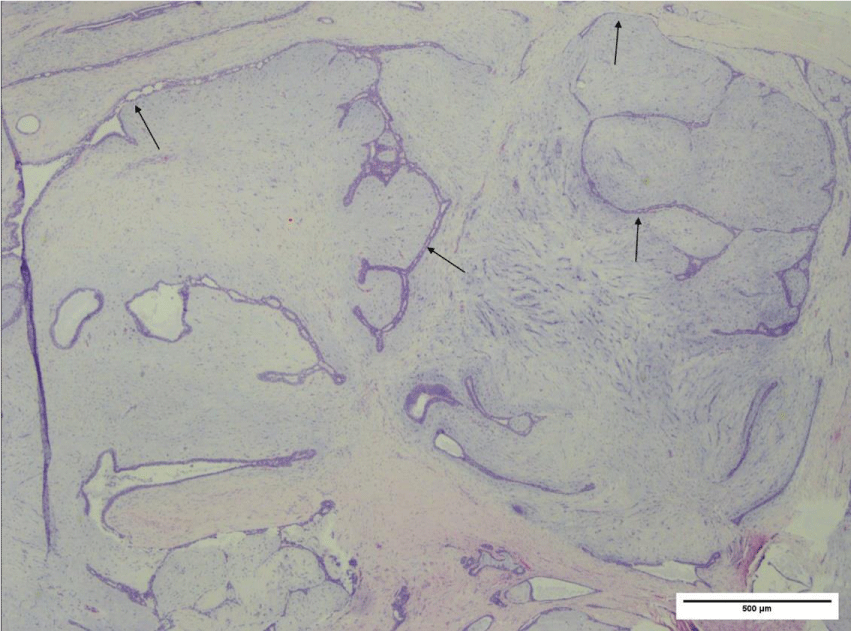
The irregular border featured focally infiltrative edges appearing as elongated epithelial-lined clefts, resulting in a “leaf-like” appearance. Overall, these features were consistent with a benign phyllodes tumor (Figure 4).
Figure 4: Photomicrograph (H&E, 2x power) of phyllodes tumor, exhibiting a biphasic neoplasm composed of benign epithelium and hypercellular stroma exhibiting a predominantly intracanalicular growth pattern with focal pericanalicular growth. Stromal proliferation results in distortion and elongation of epithelium-lined clefts (arrows).
Approximately 4 weeks later, the patient subsequently underwent an ultrasound to visualize a seroma of the prior enucleation site (Figure 5) and Savi-Scout was used to localize the area (Figure 6). The patient had an excisional biopsy performed, with no additional areas of benign phyllodes tumor identified.
Figure 5: After enucleation of the left breast mass, an ultrasound was performed to identify a seroma for SaviScout localization.
Figure 6: Successful SaviScout localization of the seroma of the prior enucleation site.
This case highlights the challenge of distinguishing fibroadenomas and phyllodes tumor radiologically and pathologically. Distinguishing between fibroadenomas and phyllodes tumors is an issue of clinical significance, as the management is different between the two diagnoses. While excisional biopsies remain the gold standard of differentiating fibroadenomas and phyllodes tumors, core needle biopsies may also provide useful diagnostic information while also reducing the need for operative management of breast fibroepithelial lesions. Although the negative predictive value of core needle biopsies for phyllodes tumors is high (93%) [5], we explore a case study of a patient with phyllodes tumor who had multiple core needle biopsy results previously consistent with fibroadenoma.
While re-excision is widely recommended for phyllode tumors after enucleation, the best way to complete a re-excision with appropriate margins of a prior enucleated mass has not been well described [13.14].
Nguyen, et al. described a case report of a fibroepithelial lesion consistent with fibroadenoma on imaging, but phyllodes tumor could not be ruled out, so a surgical excision was performed [15]. They cite that the pathologist’s suggestion of a phyllodes tumor was a helpful adjunct in determining the most appropriate surgical resection technique [15].
In the current work, we explore a case report in which multiple biopsies were consistent with fibroadenoma without the suggestion of a phyllodes tumor. Additionally, we propose a technique to obtain safe margins through the technique of Savi-Scout localization of a seroma cavity to allow for excision with 1 cm margins.
Furthermore, this case report illustrates the importance of the patient’s clinical presentation. The patient was in her 40s, and had a rapidly enlarging mass, with a lesion size of 6 cm, which are features more consistent with phyllodes tumor [4,5]. In this situation, we also explore how to achieve re-excision with negative margins after a prior enucleation for a phyllodes tumor. By performing an ultrasound for a seroma and localizing the prior cavity, a safe surgical re-excision with appropriate margins could be performed.
It is important to maintain suspicion for benign phyllodes even when approached with core needle biopsies consistent with fibroadenoma. Clinical features of the patient, including older age, fast rate of growth, and mass size greater than 6 cm should raise suspicion for phyllodes over fibroadenoma. In the event that a mass consistent with fibroadenoma is enucleated and found to be phyllodes on pathology, surgical re-excision with appropriate margins can be obtained by using ultrasound to identify a seroma cavity and using Savi-Scout localization to guide the dissection.
Ethical considerations
The informed consent of the patient was obtained prior to publication of this case report.
- Pareja F, Geyer FC, Kumar R, Selenica P, Piscuoglio S, Ng CKY, Burke KA, Edelweiss M, Murray MP, Brogi E, Weigelt B, Reis-Filho JS. Phyllodes tumors with and without fibroadenoma-like areas display distinct genomic features and may evolve through distinct pathways. NPJ Breast Cancer. 2017 Oct 12;3:40. doi: 10.1038/s41523-017-0042-6. PMID: 29043292; PMCID: PMC5638820.
- Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, Calvo JP, Dabbs DJ, Ellis IO, Eusebi V, Farshid G, Fox SB, Ichihara S, Lakhani SR, Rakha EA, Reis-Filho JS, Richardson AL, Sahin A, Schmitt FC, Schnitt SJ, Siziopikou KP, Soares FA, Tse GM, Vincent-Salomon A, Tan PH. Phyllodes tumours of the breast: a consensus review. Histopathology. 2016 Jan;68(1):5-21. doi: 10.1111/his.12876. PMID: 26768026; PMCID: PMC5027876.
- Oprić S, Oprić D, Gugić D, Granić M. Phyllodes tumors and fibroadenoma common beginning and different ending. Coll Antropol. 2012 Mar;36(1):235-41. PMID: 22816226.
- Wiratkapun C, Piyapan P, Lertsithichai P, Larbcharoensub N. Fibroadenoma versus phyllodes tumor: distinguishing factors in patients diagnosed with fibroepithelial lesions after a core needle biopsy. Diagn Interv Radiol. 2014 Jan-Feb;20(1):27-33. doi: 10.5152/dir.2013.13133. PMID: 24356293; PMCID: PMC4463252.
- Tummidi S, Kothari K, Agnihotri M, Naik L, Sood P. Fibroadenoma versus phyllodes tumor: a vexing problem revisited! BMC Cancer. 2020 Jul 13;20(1):648. doi: 10.1186/s12885-020-07129-0. PMID: 32660435; PMCID: PMC7359567.
- Duman L, Gezer NS, Balcı P, Altay C, Başara I, Durak MG, Sevinç AI. Differentiation between Phyllodes Tumors and Fibroadenomas Based on Mammographic Sonographic and MRI Features. Breast Care (Basel). 2016 Apr;11(2):123-7. doi: 10.1159/000444377. Epub 2016 Mar 23. PMID: 27239174; PMCID: PMC4881274.
- Wurdinger S, Herzog AB, Fischer DR, Marx C, Raabe G, Schneider A, Kaiser WA. Differentiation of phyllodes breast tumors from fibroadenomas on MRI. AJR Am J Roentgenol. 2005 Nov;185(5):1317-21. doi: 10.2214/AJR.04.1620. PMID: 16247156.
- Niu S, Huang J, Li J, Liu X, Wang D, Wang Y, Shen H, Qi M, Xiao Y, Guan M, Li D, Liu F, Wang X, Xiong Y, Gao S, Wang X, Yu P, Zhu J. Differential diagnosis between small breast phyllodes tumors and fibroadenomas using artificial intelligence and ultrasound data. Quant Imaging Med Surg. 2021 May;11(5):2052-2061. doi: 10.21037/qims-20-919. PMID: 33936986; PMCID: PMC8047381.
- Stoffel E, Becker AS, Wurnig MC, Marcon M, Ghafoor S, Berger N, Boss A. Distinction between phyllodes tumor and fibroadenoma in breast ultrasound using deep learning image analysis. Eur J Radiol Open. 2018 Sep 24;5:165-170. doi: 10.1016/j.ejro.2018.09.002. PMID: 30258856; PMCID: PMC6154513.
- Zhang L, Yang C, Pfeifer JD, Caprioli RM, Judd AM, Patterson NH, Reyzer ML, Norris JL, Maluf HM. Histopathologic, immunophenotypic, and proteomics characteristics of low-grade phyllodes tumor and fibroadenoma: more similarities than differences. NPJ Breast Cancer. 2020 Jun 26;6:27. doi: 10.1038/s41523-020-0169-8. PMID: 32613078; PMCID: PMC7319981.
- Komenaka IK, El-Tamer M, Pile-Spellman E, Hibshoosh H. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Arch Surg. 2003 Sep;138(9):987-90. doi: 10.1001/archsurg.138.9.987. PMID: 12963656.
- Mangi AA, Smith BL, Gadd MA, Tanabe KK, Ott MJ, Souba WW. Surgical management of phyllodes tumors. Arch Surg. 1999 May;134(5):487-92; discussion 492-3. doi: 10.1001/archsurg.134.5.487. PMID: 10323420.
- Guillot E, Couturaud B, Reyal F, Curnier A, Ravinet J, Laé M, Bollet M, Pierga JY, Salmon R, Fitoussi A; Breast Cancer Study Group of the Institut Curie. Management of phyllodes breast tumors. Breast J. 2011 Mar-Apr;17(2):129-37. doi: 10.1111/j.1524-4741.2010.01045.x. Epub 2011 Jan 19. PMID: 21251125.
- Simpson A, Li P, Dietz J. Diagnosis and management of phyllodes tumors of the breast. Annals of Breast Surgery. 2021; 5. https://abs.amegroups.org/article/view/6572
- Nguyen QD, Krider SO, Roberts JT, Posleman Monetto FE, He J. Fibroepithelial Lesion Initially Believed to Be Fibroadenoma, but Interval Growth Consistent With Phyllodes Tumor. Cureus. 2020 Sep 10;12(9):e10363. doi: 10.7759/cureus.10363. PMID: 33062486; PMCID: PMC7549856.