More Information
Submitted: September 20, 2023 | Approved: October 03, 2023 | Published: October 04, 2023
How to cite this article: Bermejo R, Perez de Heredia N, Cartagena F, Sanchez-Ferrer F, Quereda F. Fetal Ductal Constriction due to Maternal Intake of Metamizole. Arch Case Rep. 2023; 7: 046-049.
DOI: 10.29328/journal.acr.1001077
Copyright License: © 2023 Bermejo R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ductus arteriosus; Premature closure; Fetal ductal constriction; Metamizole; Right ventricular hypertrophy; Pulmonary hypertension
Fetal Ductal Constriction due to Maternal Intake of Metamizole
Rosa Bermejo1,2, Pérez de Heredia Naiara1, Faz Cartagena1,
Francisco Sanchez-Ferrer2,3 and Francisco Quereda1,2*
1Service of Obstetrics and Gynecology, San Juan University Hospital, Alicante,
Spain
2Departament of Gynecology, Miguel Hernández University, Campus of San Juan,
Alicante, Spain
3Miguel Hernandez University and Pediatric Service, San Juan University Hospital,
Alicante, Spain
*Address for Correspondence: Francisco Quereda, Service of Obstetrics and Gynecology, San Juan University Hospital, Alicante, Spain, Email: [email protected]
The prevalence of intra-uterine dysfunction of ductus arteriosus is unknown and the clinical consequences are poorly understood. We report a case of prenatal diagnosis of premature closure of the ductus arteriosus due to maternal intake of metamizole during pregnancy. Fetal echocardiography at 37 weeks of gestation revealed a right ventricular hypertrophy and suspected stenosis of the tricuspid valve. A cesarean section led to an excellent neonatal outcome. The aim of this report is to show echocardiographic abnormalities and outcomes of this rare phenomenom.
Normal fetal circulation depends on the passage of blood from the pulmonary artery to the aorta through the ductus arteriosus, which closes just after birth (in most cases during the first three days of life, although it can remain open until several months later).
The premature closure of the ductus arteriosus is a little known and infrequent entity. It is a functional fetal abnormality, which may be partial with restrictive arterial channel, or more rarely total closure, with total flow occlusion. It should be suspected in a case of fetal heart failure with or without dropsy [1,2]. In echocardiography, right ventricular hypertrophy, right cavities dilated, tricuspid and pulmonary insufficiencies, increased flow velocity in the arterial channel and pericardial effusion may be detected [1,3].
Ductal constriction usually occurs after the use of cyclooxygenase-inhibiting drugs and it is reversible after discontinuation of the medication, especially when it is diagnosed early [3,4]. It is well known that towards the end of pregnancy there is an increased sensitivity of the ductal endothelium to vasoconstrictor factors [5-7], which shows a higher incidence of ductus constriction after the 31st gestational week [4] being rare before 27 week [3] For all these reasons, we believe that the use of those drugs should be restricted in pregnant women [8], especially in the third trimester of pregnancy.
We present a 20-year-old pregnant woman controlled in our high-risk pregnancy unit due to antecedents of right ureteral stenosis operated at 2 years of age and renoureteral colics in previous gestation. Moreover, a caesarean section one year before, for placental abruption with a healthy child.
She had a complicated pregnancy. The 1st trimester screening result was of high-risk with normal 46 XX karyotype (amniocentesis), dental phlegmon intervened in 2nd trimester, urinary tract infections and recurrent renoureteral colics, especially in the 3rd trimester, so that she consults several times and she required three hospital admissions. Therefore, she needed several medications: pantomycin, fosfomycin, cefuroxime, acetaminophen, simple and compositum butylscopolamine and metamizole.
At 37th week of gestation, during a fetal ultrasound, it was detected a delayed intrauterine growth, oligohydramnios, placenta grade IV and fetal cardiopathy with very marked right ventricular hypertrophy and suspicion of tricuspid valve stenosis, with normality of rest of cardiac and fetal anatomy, as well as a cardiotocographic record. And given these findings, it was indicated to end the pregnancy by cesarean section due to unfavorable cervical conditions and previous cesarean section.
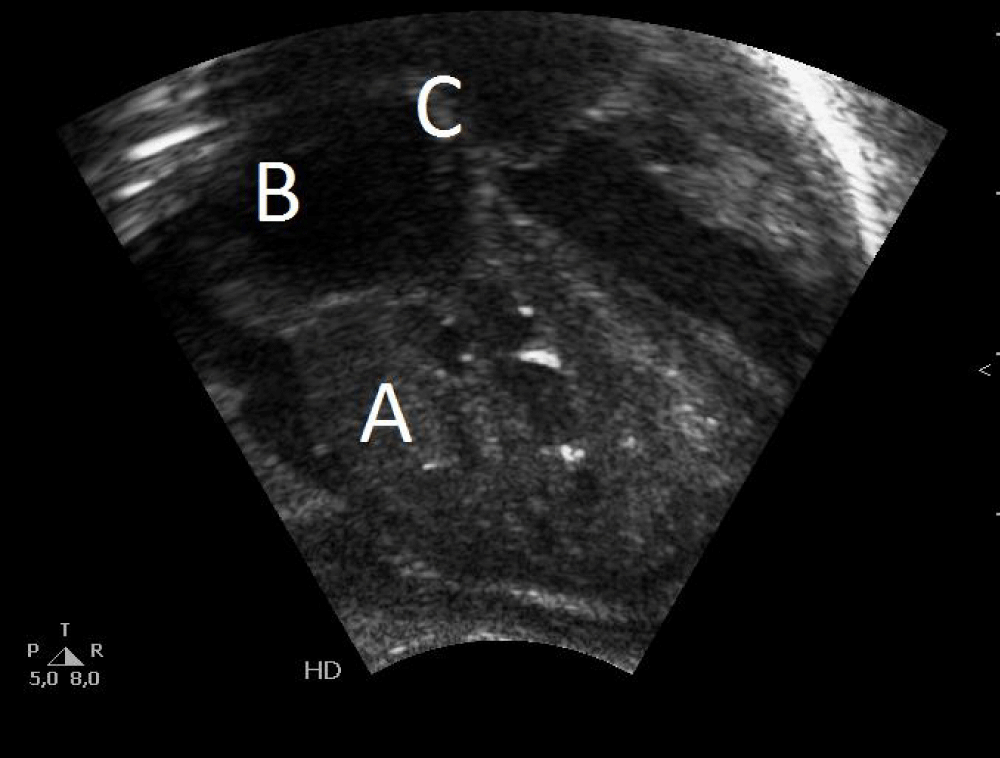
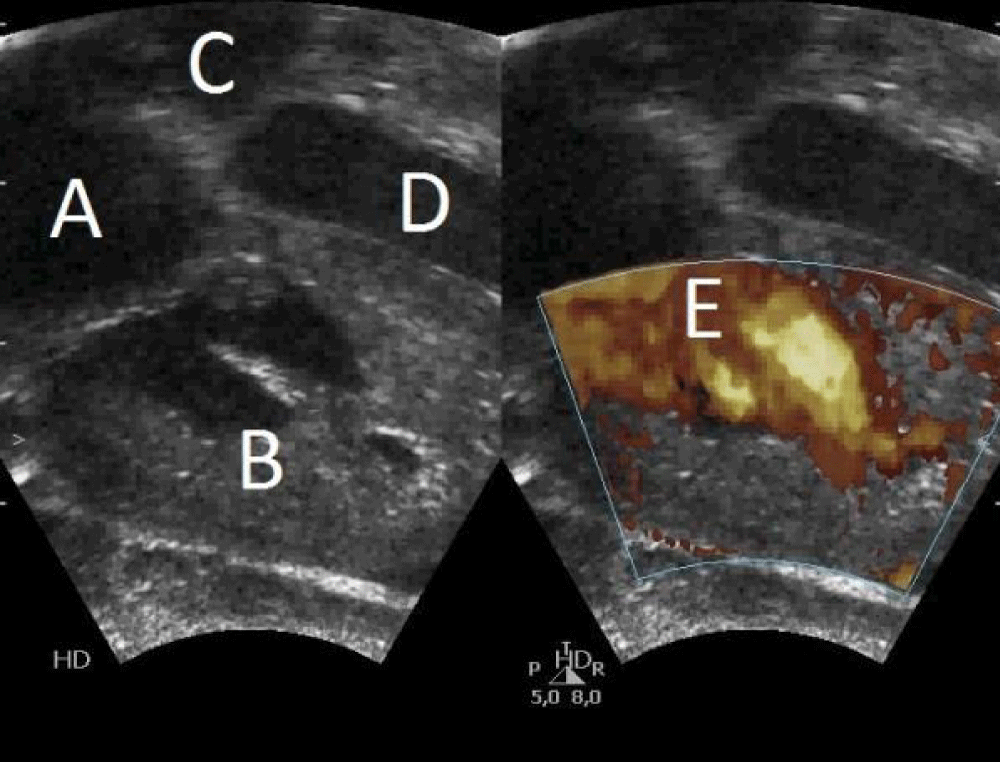
A girl weight 2400 g (percentile 12) was born, with spontaneous crying, heart rate > 100 bpm, adequate tone, and apgar 9-10. In the physical examination there weren’t pathological findings, except slight respiratory distress that require initial FiO2 30% in incubator to maintain adequate saturations. In the complementary explorations we found: an electrocardiogram with sinus rhythm at 119 bpm, right axis (+ 120º), without alterations in conduction or repolarization, with corrected QT 432 ms; an echocardiogram with non-obstructive hypertrophic cardiomyopathy of the right ventricle, patent foramen ovale, small ventricular septal defect, closed ductus arteriosus and signs of moderate pulmonary hypertension with an estimated pulmonary pressure of 55 mmHg -60 mmHg (Figures 1,2); a normal chest radiography; a normal blood test and gasometry; and a normal abdominal-pelvic and cerebral ultrasounds.
Figure 1: Postnatal echocardiography: A) Disproportionate hypertrophy of the right ventricle. B) Right atrial dilatation. C) Patent foramen ovale.
Figure 2: Postnatal echocardiography: 4-chamber section. A) Right atrium. B) Right ventricle. C) Left atrium. D) Left ventricle. E) Right ventricular filling.
She presented a favorable evolution, without vasoactive drugs or respiratory assistance, with a progressive decrease in FiO2 from 30% to 21% in 48 hours. In the echocardiographic control, a progressive decrease in right ventricular hypertrophy and decrease in pulmonary pressure was observed.
When we performed a detailed clinical history, we confirmed the antecedent of a daily maternal self-consumption of butylscopolamine and oral metamizole (dose of 575 mg every 8 hours), because of the pain caused by recurrent renoureteral colics.
Intra-uterine dysfunction of the ductus arteriosus is an acknowledged event, but it seems to be a rare phenomenon. The majority of the cases are probably subclinical or mildly symptomatic and therefore not diagnosed. In only a few cases ductal dysfunctions will come to the attention of the foetologist, neonatologist, or a paediatric cardiologist [2].
The ductus is an important structure during fetal life as it allows unloading of the right ventricle and joins the pulmonary trunk to the aorta with a diameter equivalent to these two major vessels [2,9]. The fetal right ventricle ejects 60% - 65% of the combined cardiac output, and 90% of this is shunted via the ductus to the aorta. The physiology and structure of the ductus differs considerably from the two adjacent vessels [2,7,10]. The ductus has a predominantly muscular media with circumferential fibers and a well-defined internal elastic lamina. Towards term, endothelial cushions develop which are involved with closure after birth. Prostanoids and low fetal oxygen saturations play an important role in maintaining ductal patency during fetal life [2,11,12]. The structure and shape of the ductus both vary during fetal life: initially it is quite long and then it becomes tortuous with changing of the angles of attachment to the aorta [2,13,14]. It therefore stands to reason that dysfunction of the fetal ductus may occur due to numerous factors and it may have a profound effect on the cardiovascular system [2].
So, physiological fetal circulation requires patency of the ductus arteriosus [15], and it is an active process that depends on prostaglandins [5]. As gestation proceeds, the sensitivity of the ductus to dilating prostaglandins decrease. However, the sensitivity to constricting agents as PGE-synthetase inhibitors, present in many analgesics, increase [15]. In most cases, it is related to the maternal use of non-steroidal anti-inflammatory drugs (NSAIDs) [1]. The drug that has been most frequently related to this fact is indomethacin [5], but it has been described with different medications such as ibuprofen, indomethacin, diclofenac, corticoids and, more rarely, metamizole. In addition, recent studies also show its association with the excess consumption of foods rich in flavonoids [1].
Metamizole (Nolotil®) is a non-steroidal anti-inflammatory drug that inhibits cyclooxygenase-1 and cyclooxygenase-2 activity, thereby reducing the production of prostaglandin E2 and E1. The drug is widely used in many countries as an analgesic and antipyretic agent, especially in some parts of Europe, South America and Asia [16,17].
It was banned in the United States by the Food and Drug Administration in 1977 because of a possible association with agranulocytosis. In contrast to other NSAIDs, precautions regarding the use of metamizole during pregnancy are not well defined and information on its safety in pregnancy is scarce. A weak association with Wilms’ tumor was found in children of women who took metamizole during pregnancy [16,18]. Other suggested adverse effects are leukemia and neural tube defects, that were found in mice. The association of NSAIDs with oligohydramnios was described in patients who took indomethacin [16,18], but only a few case reports had been reported associated to metamizole use [16].
While the effect of indomethacin on prenatal ductal constriction is well known, widely prescribed non-steroidal anti-inflammatory drugs such as metamizole can have an equally harmful effect [2].
Premature fetal closure of the arterial duct causes stress at different fetal ages and at many different levels of the right heart and pulmonary circulation, resulting in a wide range of secondary pathology. Disproportionate hypertrophy of the right ventricle, right ventricular and atrial dilatation, and moderate to severe tricuspid regurgitation and regurgitation of the pulmonary valve are the most frequent echocardiographic abnormalities. Pulmonary valve dysplasia associated with regurgitation may be a marker of fetal ductal dysfunction [2].
So that, the intrauterine constriction of the arterial duct causes an increase in blood flow in the pulmonary territory and a progressive increase in systolic pressure in the pulmonary artery. This maintained situation produces hypertrophy of the right ventricle, dilatation and failure of the myocardial contractility and remodeling of the pulmonary vessels, giving rise to an image of persistent pulmonary hypertension after birth [5,20].
Studies have shown evidence of important repercussions, leading to heart failure and dropsy, and it may cause fetal or neonatal death in long-term cases [3,21-24]. The delay in diagnosis can lead to persistent pulmonary hypertension in newborns, which sometimes does not respond to available therapeutic interventions [3,22,25]. Fortunately, early diagnosis allows therapeutic intervention with an improvement in prognosis. If it is associated with the use of prostaglandin inhibitors and if the causative agent is eliminated at the beginning of the clinical picture, total recovery of the alterations may occur, without evidence of neonatal complications [3,26]. However, the constriction of the ductus arteriosus may be related to serious alterations when the diagnosis is delayed, being of utmost importance the fetal monitoring by echocardiography [3,6].
In secondary cases, maternal medication should be discontinued, since spontaneous regression of the disease is possible. However, if this does not occur or if hemodynamic involvement is important, termination of pregnancy is indicated. The postnatal evolution in this situation is usually satisfactory and a rapid regression of cardiac alterations is observed, as fortunately occurred in our case with intensive care since birth to recovery [27-29].
Therefore, the decompensation of the unexplained fetal right heart requires a detailed echocardiographic evaluation of the ductus arteriosus and a detailed medical history about consum of any analgesics [15].
Long-term use of metamizol in pregnancy should always be monitored, especially in the advanced 2nd trimester and the 3rd trimester, because it may cause oligohydramnios and ductus arteriosus constriction similar to effects observed with others non-steroidal anti-inflammatory drugs.
Ethical issues
This study was carried out in accordance with the guidelines of the Declaration of Helsinki, informed consent was given by the patient, and was approved by the Ethics Committee of the University Hospital of San Juan (Alicante, Spain).
- Fariña Nogueira S, Pérez-Muñuzuri A, Couce Pico ML, López Suárez O. Cierre parcial del ductus arterioso intraútero asociado a consumo materno de flavonoides [Partial closure of the intrauterine arteriosus ductus resulting from a maternal consumption of flavonoids]. An Pediatr (Barc). 2014 Dec;81(6):e40-1. Spanish. doi: 10.1016/j.anpedi.2013.11.016. Epub 2014 Mar 2. PMID: 24598789.
- Gewillig M, Brown SC, De Catte L, Debeer A, Eyskens B, Cossey V, Van Schoubroeck D, Van Hole C, Devlieger R. Premature foetal closure of the arterial duct: clinical presentations and outcome. Eur Heart J. 2009 Jun;30(12):1530-6. doi: 10.1093/eurheartj/ehp128. Epub 2009 Apr 23. PMID: 19389789.
- Luchese S, Mânica JL, Zielinsky P. Intrauterine ductus arteriosus constriction: analysis of a historic cohort of 20 cases. Arq Bras Cardiol. 2003 Oct;81(4):405-10, 399-404. English, Portuguese. doi: 10.1590/s0066-782x2003001200007. Epub 2003 Nov 5. PMID: 14666282.
- Moise KJ Jr, Huhta JC, Sharif DS, Ou CN, Kirshon B, Wasserstrum N, Cano L. Indomethacin in the treatment of premature labor. Effects on the fetal ductus arteriosus. N Engl J Med. 1988 Aug 11;319(6):327-31. doi: 10.1056/NEJM198808113190602. PMID: 3393194.
- Arruza Gómez L, Corredera Sánchez A, Montalvo Montes J, de Marco Guilarte E, Moro Serrano M. Cierre intrauterino del conducto arterial en probable relación con la ingesta materna de metamizol durante el tercer trimestre de gestación [Intrauterine closure of the ductus arteriosus probably associated with the taking of metamizole during the third trimester]. An Pediatr (Barc). 2008 Jun;68(6):626-7. Spanish. doi: 10.1157/13123300. PMID: 18559207.
- Vermillion ST, Scardo JA, Lashus AG, Wiles HB. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol. 1997 Aug;177(2):256-9; discussion 259-61. doi: 10.1016/s0002-9378(97)70184-4. PMID: 9290437.
- Tynan M. The ductus arteriosus and its closure. N Engl J Med. 1993 Nov 18;329(21):1570-2. doi: 10.1056/NEJM199311183292111. PMID: 8413481.
- Martí Solé JJ, Pasarisas Sala M. Possible asociación entre la administración materna de metamizol e hipertensión pulmonar persistente en el recién nacido [A possible association between the maternal administration of metamizole and persistent pulmonary hypertension in a newborn infant]. An Esp Pediatr. 1996 Apr;44(4):387-8. Spanish. PMID: 8796945.
- Mott JC. Patent ductus arteriosus: experimental aspects. Arch Dis Child. 1980 Feb;55(2):99-105. doi: 10.1136/adc.55.2.99. PMID: 6769398; PMCID: PMC1626672.
- Brezinka C, Gittenberger-de Groot AC, Wladimiroff JW. The fetal ductus arteriosus, a review. Zentralbl Gynakol. 1993;115(10):423-32. PMID: 8273432.
- Van Overmeire B, Chemtob S. The pharmacologic closure of the patent ductus arteriosus. Semin Fetal Neonatal Med. 2005 Apr;10(2):177-84. doi: 10.1016/j.siny.2004.10.003. Epub 2004 Dec 15. PMID: 15701582.
- Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate. 2006;89(4):330-5. doi: 10.1159/000092870. Epub 2006 Jun 1. PMID: 16770073.
- Mielke G, Benda N. Reference ranges for two-dimensional echocardiographic examination of the fetal ductus arteriosus. Ultrasound Obstet Gynecol. 2000 Mar;15(3):219-25. doi: 10.1046/j.1469-0705.2000.00078.x. PMID: 10846778.
- Mielke G, Benda N. Blood flow velocity waveforms of the fetal pulmonary artery and the ductus arteriosus: reference ranges from 13 weeks to term. Ultrasound Obstet Gynecol. 2000 Mar;15(3):213-8. doi: 10.1046/j.1469-0705.2000.00082.x. PMID: 10846777.
- Schiessl B, Schneider KT, Zimmermann A, Kainer F, Friese K, Oberhoffer R. Prenatal constriction of the fetal ductus arteriosus--related to maternal pain medication? Z Geburtshilfe Neonatol. 2005 Apr;209(2):65-8. doi: 10.1055/s-2005-864116. PMID: 15852232.
- Weintraub A, Mankuta D. Dipyrone-induced oligohydramnios and ductus arteriosus restriction. Isr Med Assoc J. 2006 Oct;8(10):722-3. PMID: 17125127.
- Marhofer D, Jaksch W, Aigmüller T, Jochberger S, Urlesberger B, Pils K, Maier B, Likar R, Kayer B, Wallner R, Fink P, Grögl G. Schmerztherapie in der Schwangerschaft : Eine expertInnenbasierte interdisziplinäre Konsensus-Empfehlung [Pain management during pregnancy : An expert-based interdisciplinary consensus recommendation]. Schmerz. 2021 Dec;35(6):382-390. German. doi: 10.1007/s00482-021-00571-4. Epub 2021 Jul 29. PMID: 34324048; PMCID: PMC8613155.
- Sharpe CR, Franco EL. Use of dipyrone during pregnancy and risk of Wilms' tumor. Brazilian Wilms' Tumor Study Group. Epidemiology. 1996 Sep;7(5):533-5. doi: 10.1097/00001648-199609000-00014. PMID: 8862987.
- Goldenberg RL, Davis RO, Baker RC. Indomethacin-induced oligohydramnios. Am J Obstet Gynecol. 1989 May;160(5 Pt 1):1196-7. doi: 10.1016/0002-9378(89)90188-9. PMID: 2658608.
- Hofstadler G, Tulzer G, Altmann R, Schmitt K, Danford D, Huhta JC. Spontaneous closure of the human fetal ductus arteriosus--A cause of fetal congestive heart failure. Am J Obstet Gynecol. 1996 Mar;174(3):879-83. doi: 10.1016/s0002-9378(96)70317-4. PMID: 8633660.
- Downing GJ, Thibeault DW. Pulmonary vasculature changes associated with idiopathic closure of the ductus arteriosus and hydrops fetalis. Pediatr Cardiol. 1994 Mar-Apr;15(2):71-5. doi: 10.1007/BF00817610. PMID: 7997417.
- Zenker M, Klinge J, Krüger C, Singer H, Scharf J. Severe pulmonary hypertension in a neonate caused by premature closure of the ductus arteriosus following maternal treatment with diclofenac: a case report. J Perinat Med. 1998;26(3):231-4. PMID: 9773385.
- Dathe K, Frank J, Padberg S, Hultzsch S, Beck E, Schaefer C. Fetal adverse effects following NSAID or metamizole exposure in the 2nd and 3rd trimester: an evaluation of the German Embryotox cohort. BMC Pregnancy Childbirth. 2022 Aug 26;22(1):666. doi: 10.1186/s12884-022-04986-4. PMID: 36028798; PMCID: PMC9413886.
- Dathe K, Hultzsch S, Pritchard LW, Schaefer C. Risk estimation of fetal adverse effects after short-term second trimester exposure to non-steroidal anti-inflammatory drugs: a literature review. Eur J Clin Pharmacol. 2019 Oct;75(10):1347-1353. doi: 10.1007/s00228-019-02712-2. Epub 2019 Jul 4. PMID: 31273431.
- Moise KJ Jr. Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993 May;168(5):1350-3. doi: 10.1016/s0002-9378(11)90763-7. PMID: 8498410.
- Bivins HA Jr, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized trial of oral indomethacin and terbutaline sulfate for the long-term suppression of preterm labor. Am J Obstet Gynecol. 1993 Oct;169(4):1065-70. doi: 10.1016/0002-9378(93)90055-n. PMID: 8238121.
- Auer M, Brezinka C, Eller P, Luze K, Schweigmann U, Schwärzler P. Prenatal diagnosis of intrauterine premature closure of the ductus arteriosus following maternal diclofenac application. Ultrasound Obstet Gynecol. 2004 May;23(5):513-6. doi: 10.1002/uog.1038. PMID: 15133806.
- Mielke G, Steil E, Breuer J, Goelz R. Circulatory changes following intrauterine closure of the ductus arteriosus in the human fetus and newborn. Prenat Diagn. 1998 Feb;18(2):139-45. doi: 10.1002/(sici)1097-0223(199802)18:2<139::aid-pd230>3.0.co;2-#. PMID: 9516015.
- Trevett TN Jr, Cotton J. Idiopathic constriction of the fetal ductus arteriosus. Ultrasound Obstet Gynecol. 2004 May;23(5):517-9. doi: 10.1002/uog.980. PMID: 15133807.