More Information
Submitted: January 28, 2022 | Approved: April 08, 2022 | Published: April 11, 2022
How to cite this article: Susan UA, Divine AE, Frances G. Complex cyanotic congenital heart disease presenting as congenital heart block in a Nigerian infant: case report and literature review. Arch Case Rep. 2022; 6: 009-012.
DOI: 10.29328/journal.acr.1001058
Copyright License: © 2022 Susan UA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: CCHD; Congenital heart block; Case report; Nigerian infant; Literature review
Complex cyanotic congenital heart disease presenting as congenital heart block in a Nigerian infant: case report and literature review
Ujuanbi Amenawon Susan*, Amain Ebidimie Divine and Gregory Frances
Department of Paediatrics, Cardiology Unit, Federal Medical Centre, Yenagoa, Bayelsa, Nigeria
*Address for Correspondence: Ujuanbi Amenawon Susan, Cardiology Unit, Department of Paediatrics, Federal Medical Centre, Yenagoa, Bayelsa, Nigeria, Email: [email protected]
Background: The prevalence of cyanotic congenital heart diseases (CCHD) varies world wide. It accounts for a third of all congenital heart diseases. The common CCHD includes Tetralogy of Fallot(TOF), transposition of the great arteries (TGA), total anomalous pulmonary venous return (TAPVR), truncus arteriosus, and tricuspid atresia (TA). Less common variants include Ebstein’s anomaly, Hypoplastic left heart syndrome, pulmonary atresia, and single ventricle. Children of all ages can be are affected. The commonest presentation is cyanosis. Bradycardia and/or congenital heart block are rare presentations and mostly occur in the presence of an associated congenital atrioventricular block.
Case report: We report a case of a 3-month-old female presenting with congenital heart block and bradycardia at 3 months of age and found to have complex cyanotic congenital heart disease on echocardiography.
Conclusion: An infant presenting with bradycardia clinically should be screened for congenital heart defect as bradycardia may be an ominous sign of serious underlying cardiac defect.
The incidence of cyanotic congenital heart diseases (CCHD) varies worldwide. It accounts for a third of congenital heart disease [1]. CCHD can be classified into:1 cyanotic congenital heart disease (CCHD) due to right-to-left shunt with decreased pulmonary flow [tetralogy of Fallot (TOF), pulmonary atresia, right-sided hypoplastic heart], cyanotic congenital heart disease due to right-to-left shunt with the decreased aortic flow (left-sided hypoplastic heart, interrupted arch, severe coarctation) and cyanotic congenital heart disease due to bidirectional shunt (transposition of great arteries, double outlet right ventricle, tricuspid atresia).
The common CCHD includes TOF, transposition of the great arteries (TGA), total anomalous pulmonary venous return (TAPVR), truncus arteriosus, and tricuspid atresia (TA) [1,2]. Less common variants include Ebstein’s anomaly, Hypoplastic left heart syndrome, pulmonary atresia, and single ventricle. Tetralogy of Fallot has been widely documented as the most common of all CCHD [1,2]. The exact etiology of CCHD is unknown. However, some risk factors have been linked to some CCHD. Examples are chemical exposure [1,3,4] genetic, consanguinity, chromosomal syndromes such as Down syndrome, Turners syndrome, and Noonan syndrome [5], infections such as rubella in pregnancy, drug abuse in pregnancy, and maternal autoimmune disease [1,6]. Children of all ages are affected by it. Although the defect occurs in-utero, the timing of presentation may vary depending on the type of heart lesion, the severity of obstruction in the heart and/or pulmonary vasculature, and associated cardiac anomalies. For example, TGA may present early in the neonatal period while cases with TOF may not present until the end of the first half of the year. The commonest presentation is cyanosis. Bradycardia is a rare presentation and mostly occurs in the presence of an associated congenital atrioventricular block. The management of children with CCHD is multidisciplinary. Medical treatment may be combined with surgical correction of the defects or in most complex defects, inoperable. The cost implication is enormous and is usually more than anticipated. There are very few reports of complex cyanotic congenital heart disease presenting as sinus bradycardia in a child. We report a case of a 3-months-old female with complex cyanotic congenital heart disease presenting as sinus bradycardia.
We report a Baby D O, a 3 months old female neonate who was brought to Federal Medical Centre, Yenagoa by parents with complaints of fast breathing, poor suck, poor weight gain all from birth, and cough of 3 days duration. Fast breathing was present both at rest and with activity.
No history of bluish discoloration of the lips or mucous membranes. The child only lasts about 5 minutes on the mother’s breast and had a frequent interruption of feeds and thus necessitating feeding with expressed breastmilk. There was a positive history of diaphoresis. At the onset of symptoms, the mother gave syrup quinine (antimalarial).
Pregnancy was supervised in a health center. There was a positive history of maternal ingestion of alcohol-based herbal concoctions throughout the first trimester. However, there was no history of rash, fever, or autoimmune illness in the mother. Pregnancy was carried to term and delivery was via spontaneous vaginal. Cried immediately after birth, did not need resuscitation, and birth weight was 4.2 kg.
The child was being fed with expressed breastmilk and fed 6-7 times a day. She was immunized for age. She was the 5th of five children. Mother is a 37-year-old homemaker with tertiary education. Father is a 43-year-old taxi driver with secondary education. Two older siblings were macrosomic (4.0 kg and 4.2 kg). Mother is not a known diabetic or hypertensive.
There was neither history of congenital heart disease in the family nor any history of recurrent pregnancy loss or sibling death.
At presentation, she was in severe respiratory distress, moderately pale, dusky with oxygen saturation in room air of 67%, afebrile, and nil peripheral edema. Weight was 4.6 kg (77% of expected). On cardiovascular system examination, she was bradycardic with a heart rate of 54 bpm had active precordium with displaced apex beat (5th LICS lateral to MCL). She had a thrill and a left parasternal heave grade 1. Heart sounds were S1 and S2 (single S2) with a grade of 4/6 pansystolic murmur loudest at the lower left sternal border. A respiratory system examination revealed tachypnoea (RR-84 cpm) with widespread coarse crepitations. She also had tender hepatomegaly.
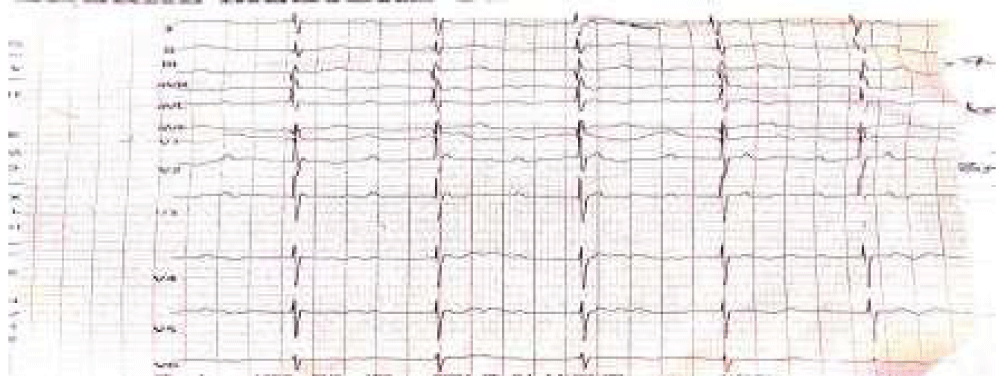
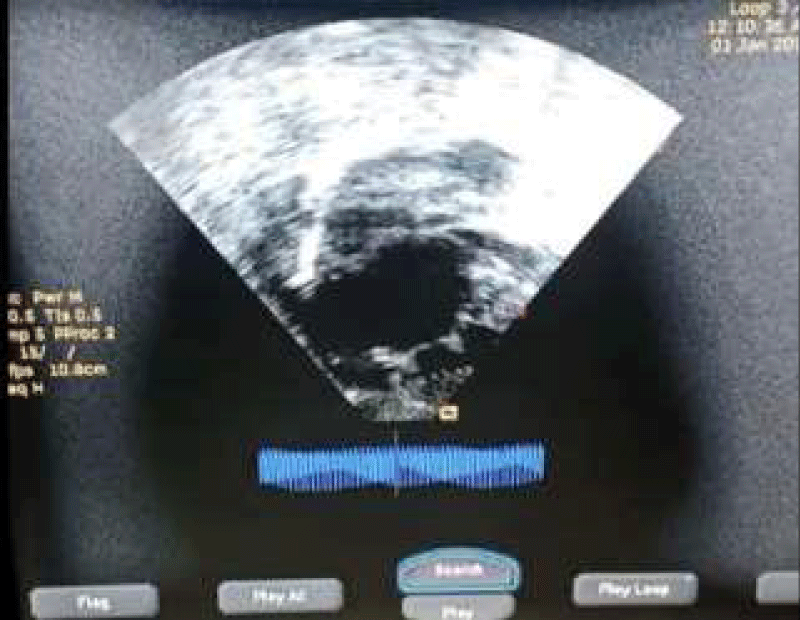
Chest radiograph (Figure 1) done showed cardiomegaly (CTR-75%) of left ventricular dominance, right atrial dilatation, and lung plethora. Twelve lead electrocardiograms (ECG) showed sinus bradycardia, prolonged PR interval (type 1 heart block), right axis deviation, and biventricular enlargement (Figure 2). Two-dimensional echocardiography (Figure 3) performed demonstrated a complex CCHD (single atrium, single ventricle pathway, complete atrioventricular septal defect-AVSD with the hypoplastic right ventricle, TGA, hypoplastic pulmonary branches, PDA, and interrupted IVC). FBC and E/U/CR + calcium were essentially normal.
Figure 1: Chest Radiograph showing cardiomegaly (CTR-75%) of left ventricular dominance, right atrial dilatation and lung plethora.
Figure 2: Twelve lead electrocardiogram (ECG) of infant showing a first-degree heart block (ECG of the infant demonstrates prolonged PR interval with sinus bradycardia)), right axis deviation, poor R wave progression and bi-ventricular hypertrophy.
Figure 3: 2D Echocardiogram –subcostal view showing a single atrium.
The child was placed on intranasal O2, intravenous antibiotics, isoproterenol, and furosemide with pacemaker implantation and cardiac surgical evaluation kept in view. However, she remained oxygen-dependent and persistently bradycardic (40-60 bpm) throughout admission. Parents signed against medical advice after 1 week of admission despite adequate counseling.
The age at diagnosis of CCHD depends on the type of heart lesion and other associated factors such as the severity of the lesion, other co-morbidity, and where the children reside (western countries or under-developed regions of the world). In a study by Animasahun, et al. [7] on cyanotic congenital heart disease among Nigerian children, it was observed that the children with TGA, TAPVC, TA, Truncus Arteriosus, and DORV were diagnosed earlier than the patients with TOF.
The average age of presentation in their study was 10 months. This was similar to other reported studies [8-11]. The fact that our patient presented at an earlier age (3 months) is not surprising as the defects were multiple and complex. In a case report by Nkoke, et al. [12] on a complete congenital heart block in a Cameroonian neonate with a complex cyanotic congenital heart defect, the age at presentation was 1 month. This neonate also had complex defects. Our patient would have presented earlier than 3 months if not for the very poor health-seeking behavior of the parents as the child was symptomatic from birth.
Our patient was a female and the case reported by Nkoke, et al. [12] was also a female. On the contrary, there was a male predominance in the study by Animasahun, et al. [7]. A few studies documented a similar report of male predominance [10,13]. While other investigators reported an equal distribution across both genders [8,11,14].
The possible associated risk factor for congenital heart defect in our patient was a maternal frequent intake of alcohol-based herbal concoctions throughout the first trimester. While in the case report by Nkoke, et al. [12] there were no maternal infections, no maternal diabetes, and no maternal alcohol consumption during pregnancy [12]. There were no clinical signs and symptoms of maternal connective tissue disease [12]. There was neither history of congenital heart disease in the family nor any history of recurrent pregnancy loss [12]. In a study by Ashraf, et al. [15] the commonest risk factors encountered among the studied neonates were positive parental consanguinity (34%), followed by maternal drug intake in 1st trimester of pregnancy (20%). Maternal infection during 1st trimester and positive family history of CCHD were found in 16% and 12%, respectively [15].
Our patient presented with fast breathing, poor weight gain, poor suck, and bradycardia.
However, obvious cyanosis was not noticed. This could be a result of the complexity of the cardiac defects with associated atrioventricular septal defect and PDA on echocardiography.
While in the case report by Nkoke, et al. [12] the patient only presented with discrete cyanosis and bradycardia. In the study by Ashraf, et al. [15] the most common presenting symptoms were central cyanosis (100%), followed by respiratory distress (82%), and feeding difficulties (62%) in the form of poor suckling, poor feeding, and interrupted feeding whereas the less common presentations were generalized convulsions (12%) and bleeding tendency (4%). This was in contrast to the study carried out by Animasahun, et al. [7] where 43.9% presented with no cyanosis. This could be due to the age of the participants studied. While only neonates were studied by Ashraf, et al. [15] Animasahun, et al. [7] studied children from 0 to 14 years. The twelve lead electrocardiogram (ECG) showed sinus bradycardia, and prolonged PR interval indicative of first-degree heart block. Congenital heart block (CHB) may be due to structural heart defects or maternal connective tissue disease or both [16]. In less than 10% of cases, congenital AV block may be either 1st or 2nd degree block at birth, of which 50% progress to 3rd degree block in postnatal life [17]. Congenital heart block can be detected in utero between 18 and 28 weeks of gestation by echocardiography in the presence of fetal bradycardia [18]. The association with a structural congenital heart defect significantly increases mortality [16]. According to Kertesz, et al. [19] in various series of fetal congenital complete atrioventricular block, 30% to 53% of cases had associated congenital heart defect [19]. Of these, only 14% survived the neonatal period compared to 85% survival of the autoimmune isolated congenital complete atrioventricular block [19].
In our index case, we were unable to test for autoantibodies due to non -the availability of facilities and limited resources. Our patient however had a structural heart defect known to be associated with congenital heart block.
Our patient had a complex CCHD (single atrium, single ventricle pathway, complete AVSD with the hypoplastic right ventricle, TGA, hypoplastic pulmonary branches, PDA, and interrupted IVC) on two-dimensional echocardiography. The patient in the case report by Nkoke, et al. [12] also had a complex CCHD (dextrocardia, a complete atrioventricular septal defect, and malposition of great arteries). In our report and the case reported by Nkoye, et al. [12] the patients had TGA and complete AVSD. In Uganda, Namuyonga, et al. [20] reported a case of isolated CHB in an infant diagnosed at the age of 7 months whereby echocardiography did not reveal any associated structural congenital heart defect.
Transposition of great arteries has been reported as the second most commonest CCHD with TOF being first [21]. On the contrary, Transposition of great arteries (D-TGA) was the most frequent type of CCHD in the study by Ashraf, et al. [15] with a frequency of 66%, followed by HLHS (12%) and complex CCHD (12%), meanwhile, the less common types were single ventricle (4%) and pulmonary atresia (4%) followed by hypoplastic right ventricle (2%) [15]. Animasahun, et al. [7] reported TGA as the third most commonest CCHD. TGA was the third most commonest in the study by Animasahun, et al. [7] because of the inclusion of isolated cases of DORV and combined cardiac lesions with DORV thereby increasing the prevalence of DORV to second [7]. In the study by Ashraf, et al. [15] only neonates were studied leading to the higher prevalence of TGA [15]. Our patient had TGA with other complex defects.
The treatment of CHB requires pacemaker implantation as well as the treatment of any underlying structural heart defect [22]. Early intervention with pacing even in the asymptomatic patient may prevent the occurrence of significant myocardial dysfunction [22]. In addition, the presence of a significant structural congenital heart defect is felt to be an indication to pace a patient with CHB [22]. Our patient could not receive pacing at the time we made the diagnosis due to the non-availability of facilities for pediatric pacemaker implantation in our facility and limited resources as there is no universal health coverage in the country for chronic diseases such as congenital heart defects. Mortality and morbidity in affected children are high.
In conclusion, the anticipation of CHB in cases of complex CCHD is important. Early detection of CHB by health care professionals and timely management is the key to the success of the infant’s survival. A thorough physical examination and an ECG can be diagnostic in patients presenting with bradycardia. For prevention, it is necessary to implement: health education and awareness creation among high-risk populations, emphasize good antenatal care and proper screening (fetal echocardiography, especially in underprivileged populations and implement proper obstetric counseling and support to the family.
- Daniel Bernstein In: R.M. Kliegman, (editor). Cyanotic Congenital Heart Disease: Evaluation of the Critically Ill Neonate with Cyanosis and Respiratory Distress. Nelson Textbook of Paediatrics. 20th ed. Philadelphia: Elsevier. 2016; 2211–2235.
- Van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, et al. Birth prevalence of congenital heart disease worldwide: a systemic review and meta-analysis. J Am Coll Cardiol. 2011; 58: 22417. PubMed: https://pubmed.ncbi.nlm.nih.gov/22078432/
- Gorini F, Chiappa E, Gargani L, Picano E. Potential effects of environmental chemical contamination in congenital heart disease. Pediatr Cardiol. 2014; 35: 559-568. PubMed: https://pubmed.ncbi.nlm.nih.gov/24452958/
- Snijder CA, Vlot IJ, Burdorf A, Obermann-Borst SA, Helbing WA, et al. Congenital heart defects and parental occupational exposure to chemicals. Hum Reprod. 2012; 27: 1510-1517. PubMed: https://pubmed.ncbi.nlm.nih.gov/22357765/
- Richards AA, Garg V. Genetics of Congenital Heart Disease. Curr Cardiol Rev. 2010; 6: 91-97. PubMed: https://pubmed.ncbi.nlm.nih.gov/21532774/
- Shawky RM, Elsayed SM, Zaki ME, et al. Consanguinity and its relevant to clinical genetics. Egyptian J Med Human Genet. 2013; 14: 157-164.
- Animasahun B, Madise-Wobo A, Kusimo O. Cyanotic congenital heart diseases among Nigerian children. Cardiovascu Diagnosis Ther. 2017; 7: 389-396. PubMed: https://pubmed.ncbi.nlm.nih.gov/28890875/
- Otaigbe BE, Tabansi PN. Congenital heart disease in the Niger Delta region of Nigeria: a four-year prospective echocardiographic analysis. Cardiovasc J Afr. 2014; 25: 265-268. PubMed: https://pubmed.ncbi.nlm.nih.gov/25388927/
- Ibadin MO, Sadoh WE, Osarogiagbon W. Congenital heart diseases at the University of Benin Teaching Hospital. Niger J Paediatr. 2005; 32: 29-32.
- Sani MU, Mukhtar-Yola M, Karaye KM. Spectrum of Congenital Heart Disease in a Tropical Environment: An Echocardiography Stud. J Natl Med Assoc. 2007; 99: 665-669. PubMed: https://pubmed.ncbi.nlm.nih.gov/17595936/
- Sadoh WE, Uzodimma CC, Daniels Q. Congenital Heart Disease in Nigerian Children. World J Pediatr Congenit Heart Surg. 2013; 4: 172-176. PubMed: https://pubmed.ncbi.nlm.nih.gov/23799730/
- Nkoke C, Wawo E, Mfeukeu L, Makamte L, Edie S, et al. Complete congenital heart block in a neonate with a complex cyanotic congenital heart defect in Africa. Cardiovasc Diagno Ther. 2016; 6: 78-82. PubMed: https://pubmed.ncbi.nlm.nih.gov/27904846/
- Asani M, Aliyu I, Kabir H. Profile of congenital heart defects among children at Aminu Kano. Teaching Hospital, Kano, Nigeria. J Med Trop. 2013; 15: 131-134.
- Chinawa JM, Eze JC, Obi I, Arodiwe I, Ujunwa F, et al. Synopsis of congenital cardiac disease among children attending University of Nigeria Teaching Hospital Ituku Ozalla, Enugu. BMC Res Notes. 2013; 6: 475. PubMed: https://pubmed.ncbi.nlm.nih.gov/24252233/
- Ashraf AT, Marwa AA, Mohamed AM. Clinical profile of cyanotic congenital heart disease in neonatal intensive care unit at Sohag University Hospital, Upper Egypt. Egyptian J Med Human Genet. 2017; 18: 47-51.
- Rosenthal E. Classification of congenital complete heart block: autoantibody-associated or isolated? Lupus 2003; 12: 425-426. PubMed: https://pubmed.ncbi.nlm.nih.gov/12873042/
- Lazzerini PE, Capecchi PL, Guideri F, Acampa M, Galeazzi M, et al. Connective tissue diseases and cardiac rhythm disorders: an overview. Autoimmun Rev. 2006; 5: 306-313. PubMed: https://pubmed.ncbi.nlm.nih.gov/16782554/
- Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J Am Coll Cardiol. 2002; 39: 130-137. PubMed: https://pubmed.ncbi.nlm.nih.gov/11755298/
- Kertesz NJ, Fenrich AL, Friedman RA. Congenital complete atrioventricular block. Tex Heart Inst J. 1997; 24: 301-307. PubMed: https://pubmed.ncbi.nlm.nih.gov/9456483/
- Namuyonga J, Lwabi P, Lubega S. Congenital heart block in a ugandan infant. Cardiovasc J Afr. 2015; 26: 28. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5119992/
- Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39: 1890-900. PubMed: https://pubmed.ncbi.nlm.nih.gov/12084585/
- Friedman D, Duncanson Lj, Glickstein J, et al. A review of congenital heart block. Images Paediatr Cardiol. 2003; 5: 36-48. PubMed: https://pubmed.ncbi.nlm.nih.gov/22368629/