More Information
Submitted: 28 May 2020 | Approved: 15 June 2020 | Published: 16 June 2020
How to cite this article: Ge S, Lou M, Zhang J, Gao G, Qu Y, et al. Ependymomas with extraneural metastasis to lung in children: A case report and literature review. Arch Case Rep. 2020; 4: 041-045.
DOI: 10.29328/journal.acr.1001039
ORCiD: orcid.org/0000-0002-5247-6727
Copyright License: © 2020 Ge S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pediatric ependymoma; Extraneural metastasis; Surgery; Radiotherapy; Poor outcome
Ependymomas with extraneural metastasis to lung in children: A case report and literature review
Shunnan Ge1#, Miao Lou1,2#, Jiarui Zhang3, Min Chao1, Yang Jiao1, Guodong Gao1, Yan Qu1 and Liang Wang1*
1Department of Neurosurgery, Tangdu Hospital, Fourth Military Medical University,
Xi’an, Shaanxi, China
2Department of Otorhinolaryngology, Head and Neck Surgery, the Second Affiliated
Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
3Department of Pathology, Tangdu Hospital, Fourth Military Medical University,
Xi’an, Shaanxi, China
#The first two authors contributed equally to this work
*Address for Correspondence: Liang Wang, Department of Neurosurgery, Tangdu Hospital, Fourth Military Medical University, 1 Xinsi Road, Xi’an City, 710038, PR China, Tel: +86 13319232049; Email: [email protected]
Ependymomas, which account for 10% of pediatric central nervous system (CNS) tumors, arise from the ependymal cells that line the cerebral ventricles and the central canal of the spinal cord. Extraneural metastasis to lung is rare for ependymomas primary tumors. Repeated surgeries that disrupt the blood-brain barrier may contribute to haematogenous spread, but the mechanism remains unclear. We present a case of ependymoma with extraneural metastasis to lung in a child and discuss reported cases of extracranial metastatic ependymoma with this presentation.
Glial neoplasms arising from the ependymal cells (ependymomas) that line the cerebral ventricles and the central canal of the spinal cord account for 10% of pediatric central nervous system (CNS) tumors [1]. Ependymomas can be graded histologically from I to III according to the World Health Organization (WHO) classification [2]. They are well demarcated from normal brain tissue but some ependymomas, especially high grade tumors, may be invasive [3]. Therefore, they have a poorer outcome compared with grade I ependymomas [4]. As a high grade tumor, anaplastic ependymomas have great potential to spread via cerebrospinal fluid (CSF) through the neuraxis. Extraneural metastasis (ENM) has been reported rarely, with extraneural metastatic sites involving lymph nodes, scalp, lung, liver and bone [5]. There are very few reports of ependymoma with ENM to lung in children. Here, we detail the first report of a supratentorial anaplastic ependymoma with extraneural metastasis to a lung in a Chinese child. We also review the literature regarding ependymomas with ENM to lung.
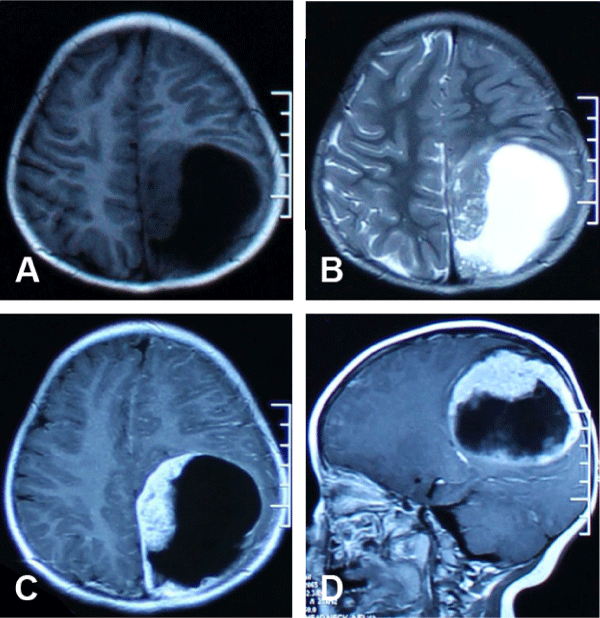
We describe a case of a 2-year-old girl, who presented in December 2014 with a history of progressive headaches and vomiting. The vomiting was a frequent early morning occurrence and was not projectile in nature. An MRI scan showed a large mixed solid-cystic supratentorial mass in the left parieto-occipital region with an associated right midline shift (Figure 1). The extraventricular mass (7.7 x 6.5 cm) had a larger non-enhancing cystic component (T1WI: homogeneously isotense, T2WI: homogeneously hyperintense) and a smaller heterogeneously enhancing solid component (T1WI: heterogeneously isotense, T2WI: heterogeneously isotense). There was a thin area of rim enhancement and no obvious peri-mass edema. Following gross total resection, histopathological evaluation demonstrated an anaplastic ependymoma of WHO grade III. The patient did not receive any chemo- or radiotherapy postoperatively.
Figure 1: The magnetic resonance images showing the lesion for the patients at initial diagnosis. (A) T1-weighted axial image. (B) T2-weighted axial image. (C) T1-weighted contrast-enhancing axial image. (D) T1-weighted contrast-enhancing sagittal image.
At 2 months postoperatively, the patient presented with further headaches and reduced consciousness, along with a bone flap bulge. A repeat CT scan demonstrated a significant outward bulge of the bone flap, and local tumor recurrence at the resection margins around the initial lesion border was confirmed via MRI. Imaging features were similar to the initial tumor, and the patient underwent a second surgical procedure with good postoperative recovery.
At 10 months after the first operation, a second tumor relapse occurred as identified by MRI scan during follow-up. The heterogeneously enhancing solid component adjacent to the midline was significantly larger than on previous occasions, with a smaller non-enhancing cystic component. For this recurrence, radiotherapy was administered. She received 59.4 Gy of craniospinal radiation in 33 fractions and no side effects or abnormalities in laboratory investigations were observed. An MRI scan after radiotherapy showed significant tumor shrinkage, with only mild residual enhancement detected. The patient recovered well to baseline function. Serial MRIs showed no evidence of intracranial recurrence over the following seven months.
In April 2016 (16 months after the initial diagnosis), the bulge in the bone flap recurred without any symptoms being reported by the patient. However, within one month, she became drowsier and MRI images revealed tumor recurrence. This time, almost all the lesion was solid, with significant heterogeneous enhancement and protrusion into the lateral ventricle. On 23 May 2016, a third surgery (the patient’s first suegery in our hospital) was performed and a gross total resection was achieved. Chemotherapy was recommended, but not accepted.
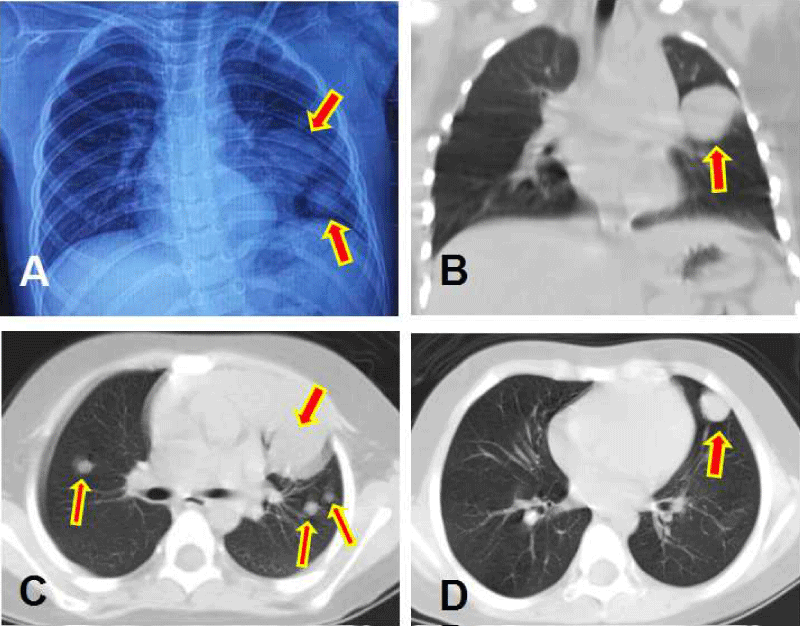
In September 2016 (21 months after the initial diagnosis), the patient again presented with headaches and nausea/vomiting accompanied by deterioration of consciousness. CT scan suggested hydrocephalus. MRI examination confirmed three portions of relapsed tumors around the border of the initial mass (left parieto-occipital, medial left occipital regions and splenium of the corpus callosum) as well as three distant recurrent lesions (left to left cerebral peduncle in the midbrain, left cerebellar hemisphere, and right fronto-parietal junction region). All of the lesions showed significant heterogeneous enhancement. The largest lesion (4.9 x 3.8 cm) was located in the left parieto-occipital region, with the adjacent dura and falx cerebrum showing significant linear enhancement. Hydrocephalus and associated interstitial brain edema, as well as brain stem compression was observed. Multiple lesions in the bilateral lung field were found in the routine preoperative anteroposterior chest radiograph. These were also seen on chest CT scan (Figure 2) and the lesions were unequal in size, uniform in density, smooth and globular in margin, and well defined with surrounding lung tissue. In addition, MRI of the spine revealed significant linear enhancement in the dura anterior to the cervical spine. The patient’s family did not agree to a pulmonary puncture biopsy to confirm the pathology of the chest lesions. Instead, the patient underwent a ventriculoperitoneal shunt procedure for palliation. Immediately after surgery, the patient’s symptoms including consciousness improved.
Figure 2: The images showing the metastasis lesions to lung, with red arrows indicating the lesions. (A) chest X- ray. (B) Pulmonary coronal CT image. (C) and (D) Two different slices of pulmonary axial CT images.
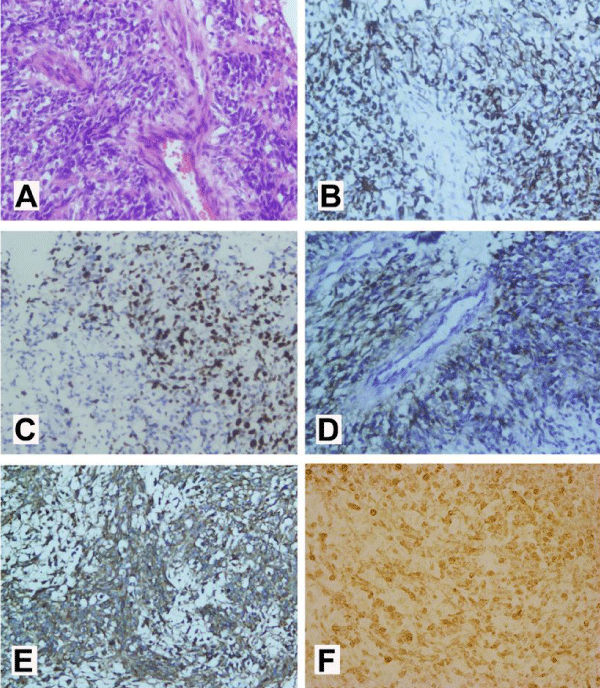
In January 2017 (25 months after initial diagnosis), the child died. No autopsy was conducted. Only the tumor tissue obtained from the surgery conducted on 23 May 2016 in our hospital was able to be described in detail in terms of the pathological evaluation, as seen in figure 3. No CSF test was performed due to parental disapproval.
A written informed consent was obtained from her father.
Figure 3: (A)Mitotic activity and perivascular pseudorosettes were conspicuous (hematoxylin and eosin, original magnification×200). (B) The tumor cells showed diffuse cytoplasmic glial fibrillary acidic protein (GFAP) immunoexpression (GFAP, original magnification×200), (C) diffuse nuclear Ki-67 immunoexpression (original magnification×200) and (D) diffuse perinuclear ‘‘dotlike’’ and ‘‘ring-like’’ epithelial membrane antigen (EMA) immunoexpression suggestive of ependymal differentiation (EMA, original magnification×200). (E) The tumor cells showed diffuse MMP-9 immunoexpression (MMP-9, original magnification×200); (F) The tumor cells showed diffuse L1CAM immunoexpression (L1CAM, original magnification×400).
In 1926, Bailey and Cushing [6] stated that distant metastases from cranial primaries do not occur. Subsequent literature has clearly challenged this viewpoint, with the reported incidence of ENM from such tumors being up to 4.3%. This figure is still substantially lower than the metastasis rate from somatic tumors to the CNS [7]. Ependymomas have a relatively better prognosis, but also have the ability to spread outside of the CNS [8]. Ali Varan, et al. [9] reported a case series involving 1011 patients with CNS tumors, and found only one ependymoma with ENM.
The PubMed database was searched for all relevant cases and case series describing ependymomas with extraneural metastasis. The following search phrases were used: “ependymomas AND extraneural”, “ependymomas AND extracranial”, and “ependymomas AND metastasis”. Results were then filtered for studies involving human subjects only. No other restrictions were applied. A total of 13 cases of ependymomas with ENM to lung in children were identified (Table 1) [5,10-20]. Thus, the clinical scenario presented in our case report is very rare and is also the first published case from a Chinese pediatric patient. There was a gender weighting towards males (10 males, 3 females).
| Table 1: Literature reporting ependymomas with extraneural metastasis to lung in children. | |||||||||||
| Authors/year | Sex/Age | Location of primary tumor | Initial histology | Surgery (times) | Radiotherapy | Chemotherapy drugs | Metastases sites | Treatment of metastasis | Number of recurrences | Duration for the first metastase after diagnosis | Overall survival |
| Wen HL, et al. (1957) | M/9 | Frontal lobe | Ependymoma | 2 | yes | none | Scalp, lungs. Cervical & hilar lymph nodes | NA | NA | NA | 1 year 22 |
| Breslich PJ, et al (1957) | M/11 | Occipital lobe | Ependymoma | 2 | yes | none | dura, lung, pleura, hilar & mediastinal lymphnodes | NA | NA | NA | 2 years 4 |
| Perrin EV, et al. (1958) | M/3 | Cerebellum | Ependymomablastoma | 2 | yes | NA | lungs, mediastinum | NA | NA | NA | 1 year 17 |
| Glasauer FE, et al. (1963) | M/2 | Parieto-occipital lobe | Ependymomablastoma | 2 (craniotomy) and 1(V-P shunt) | yes | none | lungs,lymphnodes | none | 2 | 14 months for lung, others by autopsy | 14 months 8 |
| MacMahon HE, et al (1964) | M/3 | Lateral ventricle | Malignant ependymoma | 2 | none | none | dura,lung, pleura, hilar & mediastinal lymph nodes | none | 0 | Autopsy | 15 months 13 |
| Wentworth P, et al. (1966) | M/2.5 | Parietal and occipital lobe | ependymomablastoma | 2 | 1 (XRT) | none | lung, mediastinum, hilar lymphnodes | NA | 2 | Autopsy | 12 months 23 |
| Hojgaard K, et al. (1970) | M/13 | 4th ventricle | malignant ependymoma | 2 | yes | none | lung, liver, kidney | NA | NA | NA | 9 months 10 |
| Mavroudis C, et al. (1977) | M/7 | Cauda Equina | ependymoma | 5 | 2 (XRT) | BCNU | lung | chemotherapy | 4 | 7 years | over 7 years 14 |
| Morris DM, et al.(1983) | F/10 | Cauda equina conus | Ependymoma (Grade) | 3 | 2(XRT) | none | lung, hip bone | dianhydrogalactitol,CCNU, procarbazine, cyclophosphamide and vincristine |
3 | 11 years | 12 years 15 |
| Newton HB, et al. (1992) | F/3 | Spinal cord | Ependymoma | 2 | 2(XRT) | none | Axillary lymph node, lung, liver, esophageal lymphatics, and diaphragm | Chemotherapy | 1 | Autopsy | 1 year 16 |
| M/3.5 | Supratentorial | Malignant ependymoma | 2 | 1(XRT) | CCNU | Lungs, mediastinum, thoracic lymph nodes, | none | 1 | Autopsy | 1.5 years 16 | |
| Fischer C, et al. (2012) | F/6 | Parietal lobe | Anaplastic ependymoma | 3 | 2 (XRT) | bevacizumab,irinotecan | spine, lung,liver,peribronchial and periaortal lymph nodes | tamoxifen and XRT | 3 | 27 months for spine and autopsy for others | 3 years 6 |
| Alzahrani A, et al. (2014) | M/7 | Posterior fossa | Anaplastic ependymoma | 2 (craniotomy) and 2(V-P shunt) | 2(XRT) | yes | Lung | none | 1 | 2 years | over 6 years 2 |
| Present case | F/2 | Parietal and occipital lobe | Anaplastic ependymoma | 3(caniotomy) and 1(V-P shunt) | 1(XRT) | none | Lung | none | 4 | 21 months | 25 months |
| M, male; F, female; V-P shunt, ventriculoperitoneal shunt; XRT, X-ray radiotherapy; SRS, Stereotactic radiosurgery; NA, not available for the absence of original manuscript. | |||||||||||
According to the criteria for ENM described by Weiss, [21], the lack of an autopsy to assess the lung lesions in our patient was an important limitation. It was very unlikely that a child of this young age would have suffered from a primary lung tumor and the chest radiograph 4 months before the ENM was normal. The major consideration in this case was that this was an ENM of the intracranial ependymoma.
ENM often follows repeated invasive interventions, which occurred in the present case. It has been speculated that craniotomy and shunt surgeries may contribute to metastasis by disrupting the blood-brain barrier and promoting vascular seeding to distant sites [22]. It has also been suggested that radiotherapy may interfere with the blood-brain barrier [23]. The child in current case received radiotherapy potentially adding another precipitating factor for ENM. In addition, the lesion in our case was located close to the superior sagittal sinus. Therefore, there exists the possibility of direct invasion into the vein sinus by the tumor resulting in metastasis to the lung, as has been described earlier [14].
According to the literature review, though some cases do not show typical malignant morphology such as mitosis and necrosis, a strong cell proliferative ability did occur in some cases as measured by Ki-67 index [24]. The present case showed a high Ki-67 index of 20%, which suggested a high cell proliferative ability potentially contributing to the ENM. It was notable that our patient presented with strong positive labeling for MMP, which has activity implicated in proteolysis of the extracellular matrix, regulation of cell adhesion and migration. Therefore, the histochemical features that indicate high invasiveness potential may also underline development of the ENM in our case.
We have presented a case of a 2-year-old girl who suffered from supratentorial anaplastic ependymoma, with ENM to lung only 21 months after the initial diagnosis. The patient received multiple treatments, including three craniotomies and one radiotherapy treatment. However, the prognosis remained poor, with an overall survival of only 25 months. According to the literature review, this kind of case is very rare. Further, the surgical she underwent may have disrupted the blood-brain barrier and her immune system to contribute to development of the ENM. The high Ki-67 index and strong MMP staining might be related to high invasive properties of the tumor and thus be responsible for the ENM.
- Gerstner ER, Pajtler KW. Ependymoma. Semin Neurol. 2018; 38: 104-111. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29548057
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131: 803‐820. PubMed: https://pubmed.ncbi.nlm.nih.gov/27157931/
- Khalid SI, Kelly R, Adogwa O, Carlton A, Woodward J, et al. Pediatric Spinal Ependymomas: An Epidemiologic Study. World Neurosurg. 2018; 115: e119‐e128. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29631082
- Lee JC, Sharifai N, Dahiya S, Kleinschmidt-DeMasters BK, Rosenblum MK, et al. Clinicopathologic features of anaplastic myxopapillary ependymomas. Brain Pathol. 2019; 29: 75‐84. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30417460
- Newton HB, Henson J, Walker RW. Extraneural metastases in ependymoma. J Neurooncol. 1992; 14: 135-142. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1432036
- Subramanian A, Harris A, Piggott K, Shieff C, Bradford R. Metastasis to and from the central nervous system--the 'relatively protected site'. Lancet Oncol. 2002; 3: 498-507. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12147436
- Houston SC, Crocker IR, Brat DJ, Olson JJ. Extraneural metastatic glioblastoma after interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2000; 48: 831-836. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11020581
- Rickert CH. Extraneural metastases of paediatric brain tumours. Acta Neuropathol. 2003; 105: 309-327. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12624784
- Varan A, Sari N, Akalan N, Söylemezoğlu F, Akyüz C, et al. Extraneural metastasis in intracranial tumors in children: the experience of a single center. J Neurooncol. 2006; 79: 187-190.
- Wen HL. Ependymoma with extracranial metastases. J NeuropathExp Neurol. 1957; 16: 112.
- Breslich PJ. Ependymoma of the right occipital lobe with death from extracranial metastases. J Lancet. 1957; 77: 99-103. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/13406418
- Perrin EV. Extracranial metastases from intracranial gliomata; report of two cases in children. Am J Clin Pathol. 1958; 30: 244-251. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/13571191
- Glasauer FE, Yuan RHP. Intracranial Tumors with Extracranial Metastases. J Neurosurg. 1963; 20: 474-493. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14193871
- Macmahon HE, Urista MS. The spread of an ependymoma from the brain to the lungs. Am J Surg. 1964; 107: 765-768. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14166217
- Wentworth P, Birdsell DC. Intracranial ependymoma with extracranial metastases. J Neurosurg. 1966; 25: 648-651. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/5925727
- Hojgaard K, Johansen A. A case of ependymoma with remote metastasis. Acta Pathol Microbiol Scand Suppl 1970; 212: Suppl 212: 198. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/5267090
- Mavroudis C, Townsend JJ, Wilson CB. A metastasizing ependymoma of the cauda equina. Case report. J Neurosurg. 1977; 47: 771-775. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/908942
- Fischer C, Haque SS, Huse JT, Blochin E, Souweidane MM, et al. Extraneural ependymoma: distant bone, lung, liver, and lymph node metastases following bevacizumab. Pediatr Blood Cancer. 2013; 60: 143-145. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22976578
- Alzahrani A, Alassiri A, Kashgari A, Alrehaili J, Alshaalan H, et al. Extraneural metastasis of an ependymoma: a rare occurrence. Neuroradiol J. 2014; 27: 175-178. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202865/
- Morris DM, Steinert HR, Wiernik PH. Ineffectiveness of chemotherapy in patients with metastatic ependymoma of the cauda equina. J Surg Oncol. 1983; 22: 33-36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6823114
- Weiss L. A metastasizing ependymoma of the cauda equina. Cancer. 1955; 8: 161-171. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/13231048
- Hoffman HJ, Duffner PK. Extraneural metastases of central nervous system tumors. Cancer. 1985; 56: 1778-1782. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4027909
- Adair JC, Baldwin N, Kornfeld M, Rosenberg GA. Radiation-induced blood-brain barrier damage in astrocytoma: relation to elevated gelatinase B and urokinase. J Neurooncol. 1999; 44: 283-289. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10720208
- Fujimori T, Iwasaki M, Nagamoto Y, Kashii M, Sakaura H, et al. Extraneural metastasis of ependymoma in the cauda equina. Global Spine J. 2013; 3: 33-40. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3854601/