More Information
Submitted: 17 July 2019 | Approved: 02 August 2019 | Published: 05 August 2019
How to cite this article: Gunes V, Atalan G, Bayram LC,
Varol K, Erol H, et al. Clinical, histopathological and surgical evaluations of persistent
oropharyngeal membrane case in a calf. Arch Case Rep. 2019; 3: 021-025.
DOI: 10.29328/journal.acr.1001016
Copyright License: © 2019 Gunes V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Calf; Persistent oropharyngeal membrane; Anomaly
Clinical, histopathological and surgical evaluations of persistent oropharyngeal membrane case in a calf
Vehbi Gunes1*, Gultekin Atalan2, Latife Cakir Bayram3, Kemal Varol4, Hanifi Erol2, Ihsan Keles1 and Ali C Onmaz1
1Department of Internal Medicine, Faculty of Veterinary Medicine, Erciyes
University, Kayseri, 38090, Turkey
2Department of Surgery, Faculty of Veterinary Medicine, Erciyes University,
Kayseri, 38090, Turkey
3Department of Pathology, Faculty of Veterinary Medicine, Erciyes University,
Kayseri, 38090, Turkey
4Department of Veterinary Medicine, Mehmet Akif Ersoy University, Burdur Food,
Agriculture and Livestock Vocational School, Burdur, Turkey
*Address for Correspondence: Dr. Vehbi Gunes, Department of Internal Medicine, Faculty of Veterinary Medicine, Erciyes University, Kayseri, 38090, Turkey, Tel: 00905423438007; Email: [email protected]; [email protected]
A male, 4 days old and 20 kg Simmental calf was evaluated for regurgitation and hyper salivation since birth. The mother became pregnant by artificial insemination and the pregnancy was the second of the mother. A membrane closed the pharynx and a diverticulum on dorsal of this membrane was seen during oropharyngeal examination through inspection. Membrane was also viewed by endoscopy under general anaesthesia. Larynx and oesophagus were imaged by bronchoscopy through the back side of the membrane. After these applications, it was decided that soft palate adhered firmly to the root of tongue causing congenital atresia. Surgical treatment of oropharyngeal membrane was carried out under general anaesthesia. Firstly, tracheotomy was performed for to ease breathing and membrane removed by electrocautery application. Intensive fluid accumulation and oedema formation at the incision area were detected by endoscopic examination following operation and the calf had severe dyspnoea two days after operation and died due to respiratory insufficiency. At necropsy, severe inflammatory reaction, laryngeal oedema and intensive salivation at the surgical side was determined. Direct imaging techniques should be used to determine in the closed oropharyngeal lumen. Moreover, nasopharyngoscopy should be considered to image larynx and oesophageal way. Present case is the first report with concern to pharyngeal membrane formation together with direct imaging and surgical procedures. Therefore, it was considered that this case report could be useful for colleagues and literatures.
Oropharyngeal membrane forms in two different layers from the embryonic stage. First, outher layer originates from ectodermal epitelium and the second is inner layer from the endodermal epithelium. These two layers differ to each other by a connective tissue (Deep stomodeum wall). Pharynx is closed by this tissue towards to anterior direction. Ectoderm of stomodeum forms membrana oropharyngea by fusing with blind end of foregut (Membrana buccopharenge). The membrane separates stomodeum from the front bowel, which is known as draft of mouth cavity. In the progressive stage, stomodeum and pharynx or pharyngeal membrane disappears during embryonic development for many breed in the third and fourth week of pregnancy in order to create pharynx, this provides formation of oral cavity and oropharynx following palatoglossal arch. Persistent oropharyngeal membrane separates oral cavity from oesophagus and laryngeal entrance and then stays permanent because of insufficiency of embryonic development of gastrointestinal tract. Briefly, it is a developmental anomaly of oropharynx [1-8].
First day after administration
A male, 4 days old and 20 kg Simmental calf was referred to Erciyes University, School of Veterinary Medicine Clinics at 11 April 2016. The calf had regurgitation (milk could not be swallowed so directly came out from the mouth) and hypersalivation since birth. It was second calving of the cow, which became pregnant with artificial insemination. General condition of the calf was weak but mucosal colour was normal and had mild level of dehydration. Body temperature, respiratory rate and pulse frequency were 38.7 oC, 44/min and 92/min, respectively. A commercial Bovine Viral Diarrhoea Virus (BVDV) antigen kit (BVDV Ag Point-of-Care-Test, Idexx) were used for the presence of BVD virus.
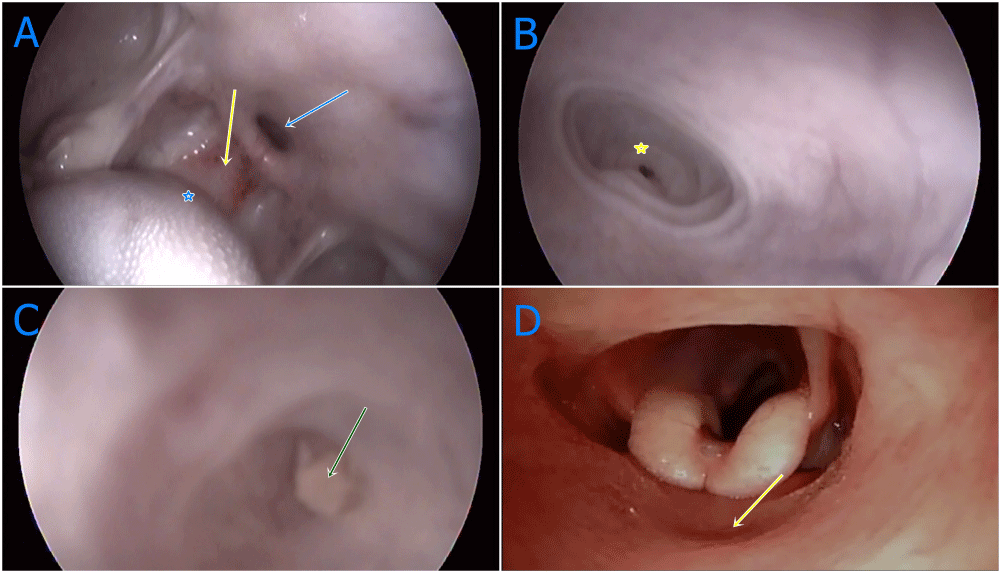
In the first evaluations of the calf, there was a complaint of regurgitation and therefore, oesophageal tubing made to relieve possible obstruction via mouth but no transition observed. Therefore, detailed pharynx examination was carried out under general anaesthesia by an endoscopy device (Eickemeyer Eickview 150 LED videoendoscope). For general anaesthesia, 0,1mg/kg xylazine hydrochloride (Xylazine hydrochloride, Basilazin %10, Bavet Ilac San. and Tic. A.Ş Tepeoren-Tuzla Istanbul) injected intramuscularly for sedation, 5 minutes after 1mg/kg ketamin hydrochloride (Ketamin hydrochloride, Ketasol %10 Richter Pharma AG, Wels Austria) injection intravenously. Front side of oropharyngeal membrane, dorsal to diverticulum was viewed by endoscopy. Back side of the membrane, larynx and oesophagus were imaged by a bronchoscop (Eickemeyer Eickview 70 LED bronchoscope). At the endoscopic examination, soft palate was viewed which was adhered firmly to the base of tongue causing congenital atresia (Figure 1A). There was a blind pouch access approximately 3 mm in width at the dorsal aspect of atresia (Figure 1B). The blind pouch was enlarged by free air during endoscopy. The pouch quite narrowed and obstructed by milk clot (Figure 1C). Using a suitable size of bronchoscope, isthmus faucium was imaged entering from nostril and nasal space, larynx, oesophageal and tracheal structures were recorded (Figure 1D). Nasopharyngoscopy and endoscopy were applied together following the procedures. The catheter could be passed through the natural hole which it was 3 mm in width, located in the oropharynx, extending to Isthmus faucium. No important findings were encountered at mouth and oropharyngeal junction apart from atresia by physical examination.
Figure 1 A-D: Oropharengeal and nasopharengeal endoscopic images of oropharangeal membrane, A: General appearences of oropharenx with congenital anomaly. Yellow arrow; congenital oropharengeal membran, blue arrow; diverticulum dorsal to oropharengeal membran, blue star; caudal of tonque, B: Near plan image of diverticulum inflated with air. Yellow star; blind sac at the end of diverticulum, C: Coagulated milk accumulation at the end of blind sac (green arrow), D: Broncoscop application via nasal approach, larynx imaging at back of oropharengeal membran (yellow arrow), intermediate wall of oropharengeal membran.
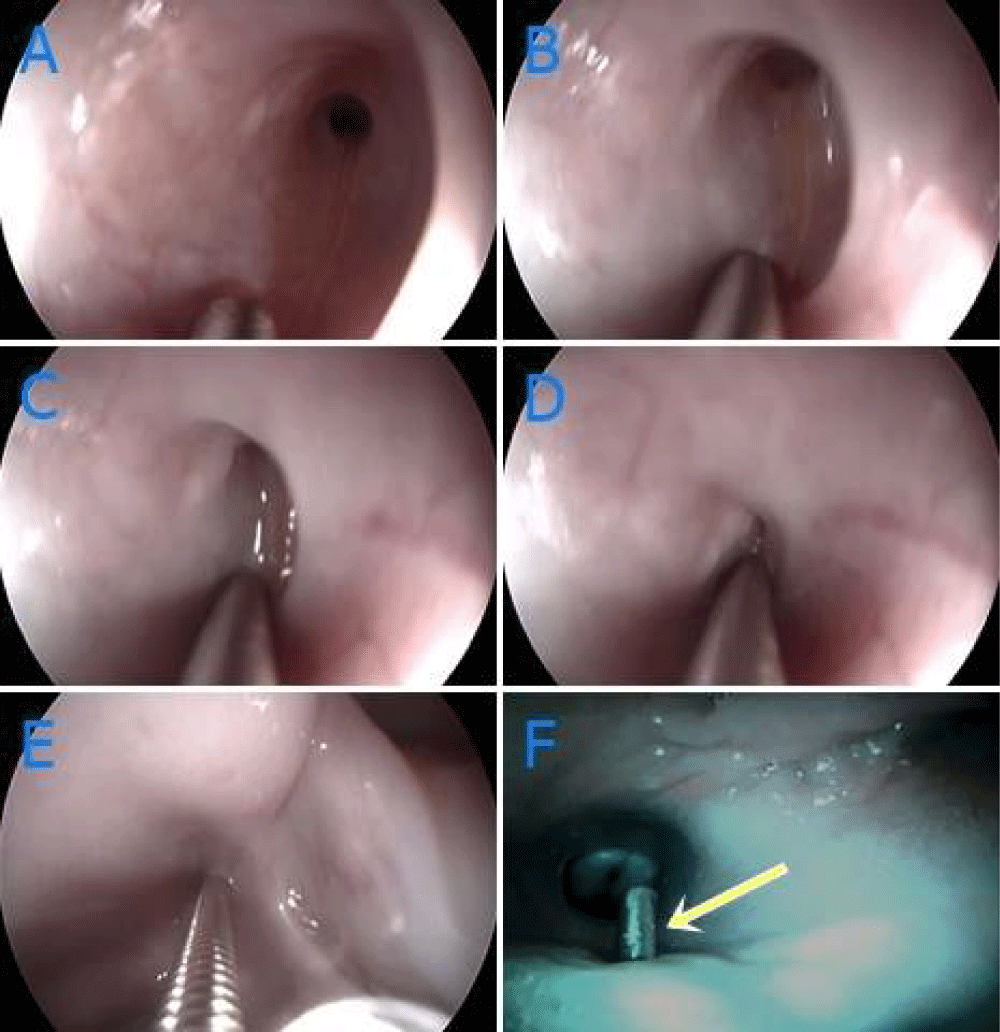
There was a swallowing reflex during catheterisation when a metal forceps touched to the membrane (Figures 2A-F).
Figure 2 A-F: Examination of diverticulum by endoscopy and bronchoscopy, A-E: Contraction occured by contacting of guide to mucosa, F: Imaging of guide protruding from intermediate of blunt sac (yellow arrow).
Blood sample with ethylenediaminetetraacetic acid (EDTA) was used for hematologic analyses via a hematology analyzer (Mindray BC 2800 Vet. Auto Hematology Analyzer).
After diagnostic imagings, an oesophageal feeding tube was placed to feed the animal and to help the animal to get better its general condition before operation. For this purpose; sedation (Xylazine 2% 1 ml / 100 kg IM) was applied. The calf was then laid in the right lateral position and local anesthesia (Lidocaine 2%, 10 ml) was infiltrated from the left sulcus jugularis. Then, 5 cm skin incision was made and the surrounding tissues and trachea were retracted and the esophagus was observed. Approximately 1 cm vertical incision was made in the esophagus, then, esophageal tube (Properties) was placed and fixed, the skin was closed with simple separate sutures. The calf was then fed through this tube. In addition, coagulated milk pieces detected during tubing which considered originated from natural hole.
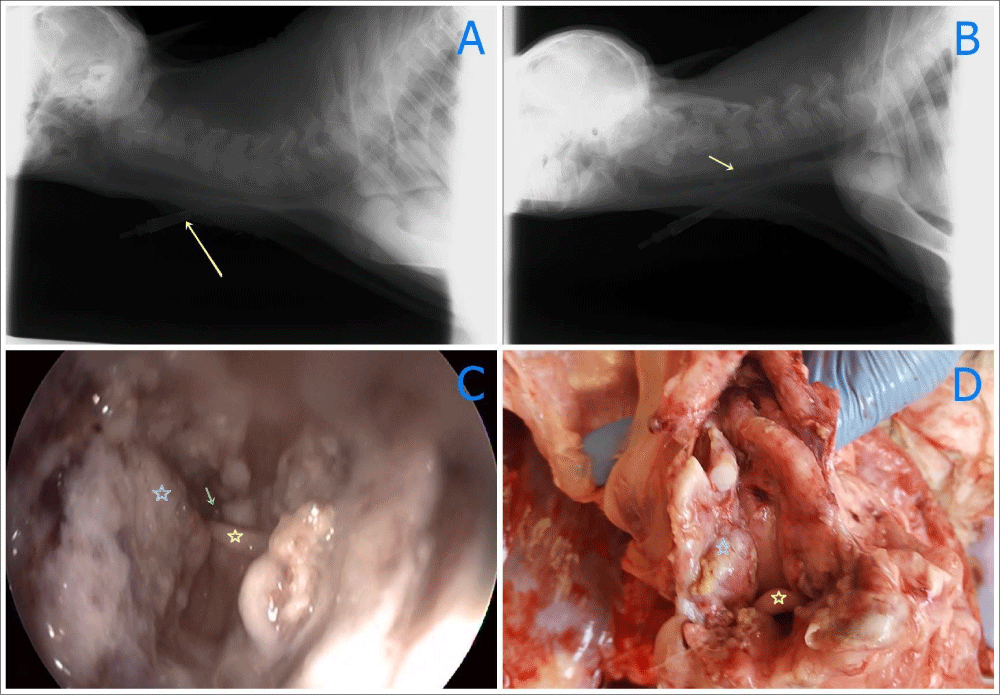
Radiographic examination was carried out to see the oropharyngeal membrane (Fuji Film Computed Radiography CR-IR 392). But oropharyngeal membrane was not seen on plain X-ray. The oesophageal tube was also viewed in the radiographic examination (Figure 3A).
Figure 3 A-D: Radyographic, endoscopic and necropsy findings before surgery (yellow arrow), A: Radyographic findings before surgery B: Radyographic findings following surgery: some pus in the trachea (yellow arrow) C: Endoscopic imaging after one day of surgery (blue star); Incised part of oropharangeal area (yellow arrow); Larinx (green arrow); fluid in larynx, D: View of pharengeal side in necropsy (yellow star), larinx (blue star), incised part of oropharengeal area.
Before making operation, fluid disturbances were corrected by 0.9 % NaCl2.
Second Day after administration, a temporary tracheotomy was applied to relieve respiration before operation.
For general anaesthesia; 0,1 mg/kg xylazine hydrochloride injected intramuscularly for sedation, 5 minutes later, 1mg/kg ketamin hydrochloride injected intravenously and anaesthesia maintained by 2% sevoflorane (Sevorane, Aesica Quccnborough Ltd. Quccnborough Kent ME 11 5HL, England). After general anesthesia, the calf was turned to the ventrodorsal position for tracheotomy, approximately 5 cm of skin incision was made at the level of the 6-8 tracheal rings from the ventral line of the neck, subcutaneous connective tissue and the region was seen. The annular ligament between ring 7 and 8 was then incised transversally and the intubation tube (12 mm, Kruss, Germany) was inserted. The calf was connected to the general anesthesia device. After the operation, the intubation tube was removed and the operation site was closed by known routine technique.
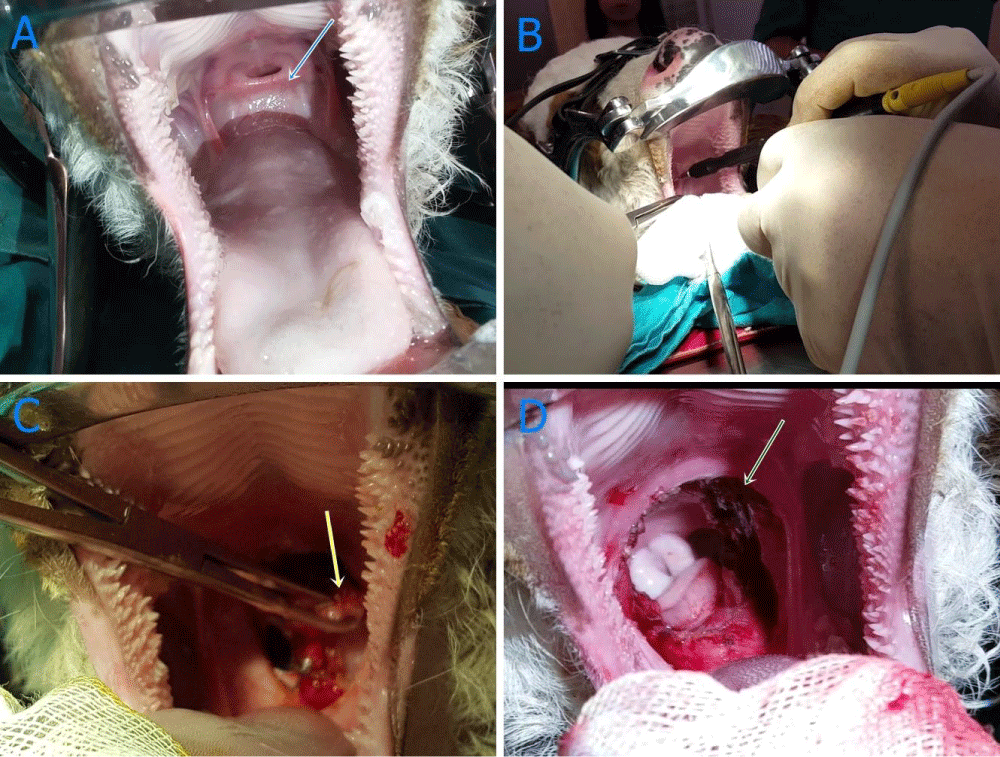
Just after Tracheatomy, the membrane was removed surgically by making circumferential incision by an electrocautery (Elektro-Mag M 20-40, electrosurgical unit monopolar and bipolar) under general anaesthesia. Circumferential resection of oropharyngeal membrane was carried out (Figure 4A-D). Tracheal tube was removed following operation since a smooth respiration was monitored. Radiography was taken following operation and endoscopy and nasopharyngoscopy made one day after operation (Figure 3B). The contrast plain X-Ray film showed the some opacity on pharynges and pus aspirated in the trachea. Intensive fluid accumulation and oedema were recorded on the operation area by endoscopy (Figure 3C). After operation, white blood cells (WBC) count (106/l), lymphocyte (109/l), monocyte (109/l), red blood cells (RBC) count (109/l), haemoglobin concentration (g/dl) and haematocrit (PCV) (%) values decreased compared to those of initial values, which were also within normal reference ranges (Table 1). Two days after surgery, dyspnoea occurred and animal died on 2 days after operation.
Figure 4 A-D: Imaging recorded during surgery. A: Before surgery (blue arrow); oropharangeal membran, B: Extraction by electrocoter (yellow arrow); oropharengeal membran, C: Removing of membran by electrocautery, D: complete removing of membran following surgery (green arrow).
| Table 1: Haematologicial findings before and after operation. | ||||
| Haematologicial Findings | Units | Before operation | After operation | Reference Interval [13-16]. |
| WBC | ×109/l | 4.4 | 3.2 | 3.4-9.6 |
| Lymphocytes | ×109/l | 2.4 | 0.9 | 1.9-5.9 |
| Monocyte | ×109/l | 0.4 | 0.2 | 0.2-0.8 |
| Granulocyte | ×109/l | 1.6 | 2.1 | 3.80±0.77 |
| RBC | ×1012/l | 7.29 | 6.33 | 1.49-7.63 |
| HGB | g/dl | 10.8 | 8.9 | 2.21-10.41 |
| PCV | % | 31.4 | 27.8 | 23-31 |
| MCV | fL | 43.2 | 44.0 | 3.5-42.8 |
| MCH | pg | 14.8 | 14.0 | 11.0–17.0 |
| MCHC | g/dl | 34.3 | 32.0 | 36.0-40.0 |
| PLT | 109/l | 832 | 454 | 212-451 |
| Abbreviations: (HGB) haemoglobin, (MCH) mean corpuscular haemoglobin, (MCHC) mean corpuscular haemoglobin concentration, (MCV) mean corpuscular volume, (MPV) mean platelet volume, (PCV) Haematocrit, (PLT) platelet, (RBC) Red blood cells, (WBC) White blood cells. | ||||
Four days after administration, the animal died and immediately necropsy was performed. In necropsy, severe inflammatory reaction, oedema and excessive salivation at the side of operation area were noticed. Because of this, it was believed that the animal died due to asphyxia and sepsis depending on laryngeal oedema observed at the tracheal entrance (Figure 3D). In pathological findings, macroscopic inspection of the oral cavity showed a fold of tissue extending from the soft palate to the tongue, because of this, a complete partition between the oral cavity and oropharynx found to be formed. No other grossly observable congenital defects or lesions were present (Figure 3D). Tissue samples obtained during surgery and autopsy at oropharyngeal area were fixed in formaldehyde for 24 hours and blocked in paraffin after exposed to alcohol and xylene. Four to five µm thick sections were stained with haematoxylin and eosin (H&E) and examined microscopically for histopathological changes. Digital camera (Olympus DP71) and digital programmers (DP Controller and the DP Manager) fitted to a microscope (BX-51, Olympus) (minimum of 4 fields per slide, using x10, x40) were used to photograph all slides.
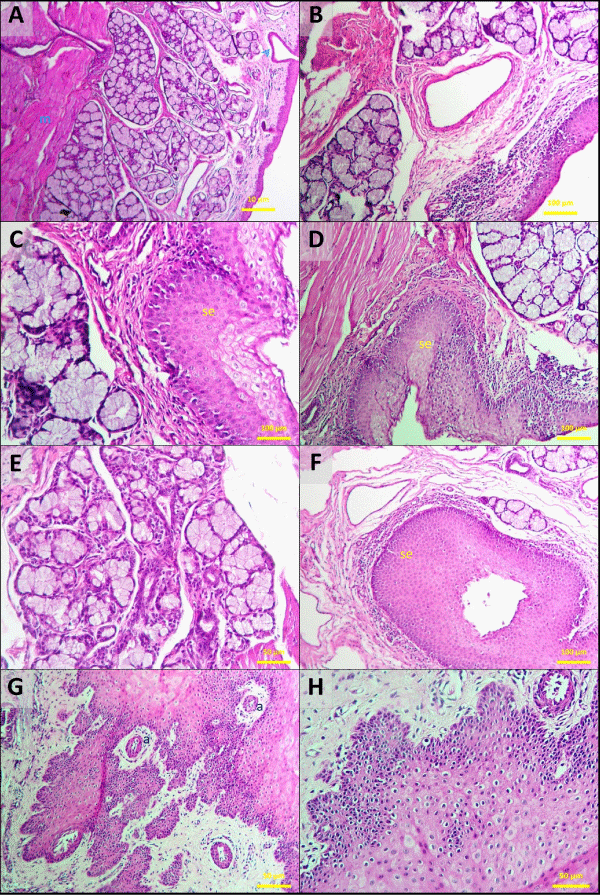
The abnormal membrane that lies between the mouth and the soft palate found to be covered with stratified squamous epithelium. The mucosa of soft palate coated with stratified flattened and non-cornified epithelium. The soft palate mucosa cell layers were in lower numbers at oropharyngeal area. The surface was covered with non-cornified, pluristratified irregular surface and flattened epithelial cells having papillary extensions. The stratum basale layer consisted of cells having 1 or 2 eccentric nuclei, condensed chromatin clusters, round, oval shape. Stratum spinosum, elongated more towards superficial epithelium had different numbers of cells, stained lightly and had big nuclei. Cytoplasm of the elongated and degenerated cells were strongly eosinophilic, and their nuclei stained darkly. Mucous glands in the propria and in the lymphoid tissue and submucosa muscle layer were present (Figure 5A-H).
Figure 5 A-H: Oropharynx. Mucous membrane with stratified squamous epithelium. This is the mucosa of the oropharyngeal aspect of the soft palate. the connective tissue papillae (a) are of moderate/high height and the stratified squamous epithelium (se) is not keratinized. Mucus secreting glands open onto the surface (arrowed). Propria-submucosa contains mucous glands (B) and lymphoid tissues. Muscularis externa (m), formed by skeletal muscle fibers. H&E stain; bar = 10 μm (A), bar = 50 μm (E-H), bar = 100 μm (B,C,D,F).
Permanent status of oropharyngeal membrane is a rare defect that defined in a human patient [8,9]. Such anomaly is also reported first time in a Hereford calf in 1996 by Smoak and Hudson [8]. In this case, the calf was 4 days old which had a tissue covering caudal of oral cavity was encountered and the case evaluated by imaging techniques and necropcy [8]. Literatures regarding to the oropharyngeal membrane in veterinary medicine are insufficient. It has been reported in brachyocephalic dogs due to similarity of choanal atresia, moreover, a permanent membrane covering oropharynx also defined [10]. This kind of congenital anomaly prevents passage of milk and the other foods to oesophagus.
The present study indicates different clinical finding from that of the reported by Smoak and Hudson [8]. The calf in the present study defecated and regurgitated milk in contrary to reported by Smoak and Hudson [8]. Furthermore, a small amount of milk came out following oesophagostomy in the present case. A significant loss of body condition was also reported for the animal with oropharyngeal membrane. The birth weight and body condition of the calf in our study was also low as reported in the literature [8]. Although, aetiology of persistent oropharyngeal membrane has not been known completely yet. Pregnancy, some uncertain effects or viral infection agents may lead to the disorder [8,9]. Thus, a commercial Bovine Viral Diarrhoea Virus (BVDV) antigen kit (BVDV Ag Point-of-Care-Test, Idexx) were used for the presence of BVD virus but the result was negative.
Hematological analyses results obtained after initial examination were evaluated within normal references ranges (Table 1). In studies on normal hematologic profile of neonatal calves, it is observed that their values are quite different and in wide ranges [11-16]. In this case, preoperative and postoperative hematological values were found to be very close to the reference values obtained from previous studies on calves of different breeds and genders. In a recent study by Panousis et al. (2018) investigated Median hematological profiles of 254 Holstein calves aged between 1-9 days and in the present study both pre- and post-operation haematological values were within the Min-Max values of above study [16]. However, postoperative WBC, lymphocyte, monocyte, RBC, hemoglobin, PCV, MCHC and PLT values were found to be lower than the same values obtained before operation. It was supposed that the decrease in the blood values might be the result of bleeding during and after the operation.
Oropharyngeal membrane may occur due to genetic or tetragenic reasons [8]. However, this hypothesis is still not clear due to unproved evidences. The most common face and mouth anomalies in animals are; agnathia, brachygnathia, choana atresia or stenosis, naso lacrimal atresia, aglossia and abnormal teeth developmental disorders [2].
Oropharyngeal membrane is an uncommon type of congenital defect. Membrane resection has been made for the treatment in human and dog [12], but no treatment has been reported in farm animals. Surgical intervention was experienced first time in this case.
Radiographic examination revealed restricted clues in this case. Oropharyngeal cavity could not be observed clearly by X-ray, but the opacities due to pus in the trachea and postoperative prognosis after operation was determined. Endoscopic examination of diverticulum should be made in the case of persistent oropharyngeal membrane via the mouth. Nasopharyngoscopy application may also be required to image larynx-oesophagus entrance, epiglottis and back of closed oropharyngeal cavity. It helps to provide valuable information for evaluation of multiple anomalies and detailed image of anatomic structures. This types of anomalies cause difficulty for veterinarian regarding management and treatment. Calf may prone to infection and die quickly because of having insufficient colostrum and passive transfer insufficiency. Moreover, in the present case report, although all precautions {hygienic precautions and antibiotic (Reptopen S, 1ml/10 kg IM, CEVA, Turkey) and Nonsteroidal Antienflammatory Drug (Fulimed, 2ml/45 kg IM, Alke, Turkey) for 2 days after operation} for the health of the calf were taken, the animal had diarrhoea and local infection following operation, indicating passive transfer insufficiency.
Supporting of immune system should be maintained before operation for such animals. It was supposed that another reason of death was tracheal aspiration of exudates (Figure 3B). Therefore, tracheal tube placement should be left postoperatively until improvement of general body condition.
In conclusion, oropharyngeal atresia encountered in this study differed from that of the previous reported case [8]. Imaging, surgery and histopathological examination were carried out first time in this report on contrary to the other study [8]. The study was considered useful for veterinarian and literature. Electrocautery, cryosurgery and laser surgery should be used to minimize possible bleeding.
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
- Hyttel P, Sinowatz F, Vejlsted M, Betteridge K: Essentials of domestic animal embryology. Saunders, Elsevier. 2009, 246-304.
- McGeady TA, Quinn PJ, Fitz Patrick ES, Ryan MT. Veterinary embryology. John Wiley & Sons, Blackwell Publishing. 2013, 205-287.
- Ramachandran S, Panchaksharappa MG, Annigeri RG, Shettar SS. Persistent buccopharyngeal membrane in an adult an incidental finding–A case report. Int j dent clin. 2010; 2: 74-76.
- Verma S, Geller K. Persistent buccopharyngeal membrane: Report of a case and review of the literature. Int J Pediatr Otorhinolaryngol 2009; 73: 877-880.
- Tan S, Tan H, Lim C, Chiang W. A novel stent for the treatment of persistent buccopharyngeal membrane. Int J Pediatr Otorhinolaryngol. 2006; 70: 1645-1649.
- Kara C, Kara I. Persistent buccopharyngeal membrane. Otolaryngol Head Neck Surg. 2007; 136: 1021-1022. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17548001
- Latshaw WK. Veterinary Developmental Anatomy. BC Decker, Philadelphia, PA. 1987; 87-126.
- Smoak IW, Hudson LC. Persistent oropharyngeal membrane in a Hereford calf. Vet Pathol 1996; 33: 80-82. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8826010
- Ooi EH, Khouri Z, Hilton M. Persistent buccopharyngeal membrane in an adult. Int J Oral Maxillofac Surg. 2005; 34: 446-448. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16053859
- Fubini SL, Ducharme N. Farm animal surgery. Elsevier Health Sciences, 2004, 226.
- Aiello SE. Hematologic Reference Ranges. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ., USA. 2017; http://www.merckvetmanual.com/mvm/appendixes/reference_guides/hematologic_reference_ranges.html#v3362733.
- Longacre JJ. Congenital atresia of the oropharynx. Plast Reconstr Surg (1946). 1951; 8: 341-348. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14891381
- Klinkon M, Ježek J. Values of blood variables in calves. In: A Bird’s-Eye View of Veterinary Medicine. Slovenia, Intech. 2012; 301-320.
- Brun Hansen HC, Kampen AH, Lund A. Hematologic values in calves during the first 6 months of life. Vet Clin Pathol 2006; 35: 182-187. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16783710
- Marutsova V, Binev R, Marutsov P. Comparative Clinical and Haematological Investigations in Lactating Cows with Subclinical and Clinical Ketosis. Mac Vet Rev. 2015, 38: 2159-2166.
- Panousis N, Siachos N, Kitkas G, Kalaitzakis E, Kritsepi-Konstantinou M, et al. Hematology reference intervals for neonatal Holstein calves. Res Vet Sci. 2018; 118: 1-10. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29331737