More Information
Submitted: October 10, 2025 | Approved: October 31, 2025 | Published: November 03, 2025
How to cite this article: Arthi J, Bagyalakshmi J. Effect of Cabbage Extract on Paracetamol Dissolution: An in-vitro Drug-food Interaction Study. Arch Case Rep. 2025; 9(11): 346-349. Available from:
https://dx.doi.org/10.29328/journal.acr.1001172
DOI: 10.29328/journal.acr.1001172
Copyright license: © 2025 Arthi J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cabbage; Paracetamol; Drug-food interaction; in vitro; Dissolution
Effect of Cabbage Extract on Paracetamol Dissolution: An in-vitro Drug-food Interaction Study
Arthi J1 and J Bagyalakshmi2*
15th Pharm-D Student, Sri Ramakrishna Institute of Paramedical Sciences, College of Pharmacy, Coimbatore – 641044, India
2Professor of Pharmaceutics, Sri Ramakrishna Institute of Paramedical Sciences, College of Pharmacy, Coimbatore - 641044, India
*Address for Correspondence: J Bagyalakshmi, Professor of Pharmaceutics, Sri Ramakrishna Institute of Paramedical Sciences, College of Pharmacy, Coimbatore - 641044, India, Email: [email protected]
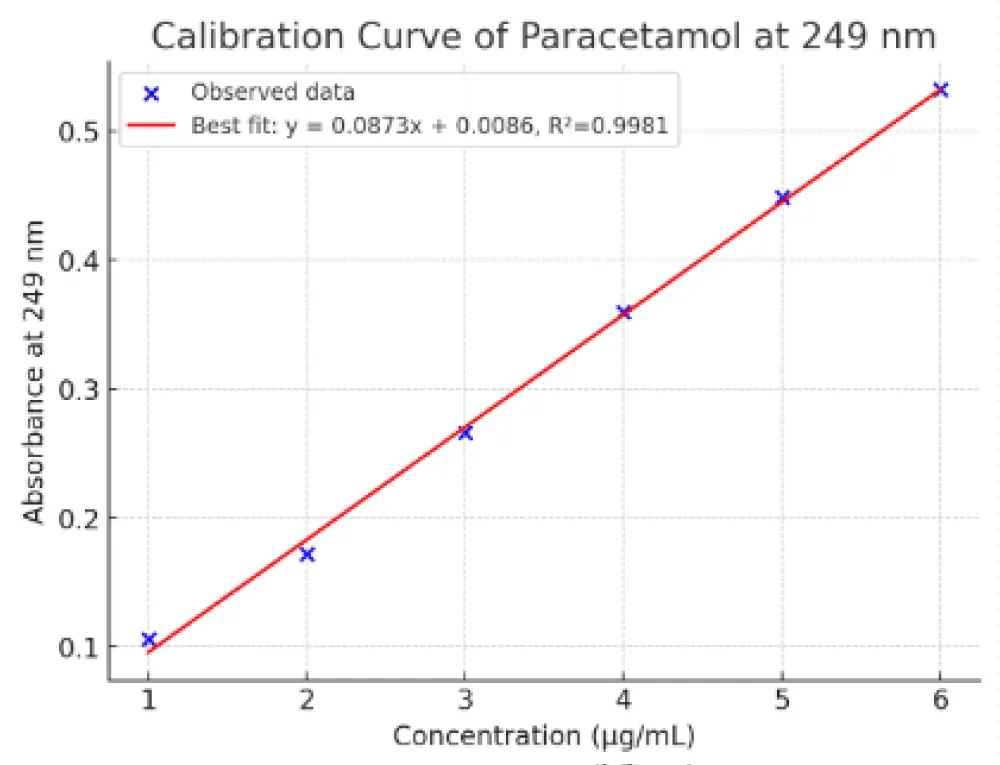
The interaction between food components and pharmaceutical agents can significantly influence drug dissolution and absorption. The present study aimed to investigate the potential in vitro interaction between cabbage extract and paracetamol using UV spectrophotometry and dissolution testing. A calibration curve for paracetamol was established at 249 nm over the concentration range of 1–8 µg/mL. The curve demonstrated linearity with an R² value of approximately 0.998, confirming that the drug follows Beer–Lambert’s law and validating the method for quantitative estimation.
Dissolution testing of a marketed paracetamol tablet was carried out in phosphate buffer at pH 5.8, simulating intestinal conditions. Absorbance values were recorded at 15-minute intervals up to 75 minutes, and the cumulative percentage drug release was calculated. The paracetamol control group exhibited a predictable, linear dissolution profile with a steady increase in absorbance, consistent with expected release behaviour. In contrast, the test group containing cabbage extract, prepared by aqueous maceration and introduced directly into the dissolution medium, demonstrated a non-linear release pattern. Although the absorbance values remained within the standard range (≤ 0.8), noticeable deviations from the control profile were observed, suggesting possible interference.
The observed variation may be attributed to phytochemicals present in cabbage, which could alter paracetamol solubility or affect UV spectrophotometric detection. Pharmacokinetic parameters were further evaluated using a one-compartment model, supporting the notion of altered dissolution dynamics in the presence of cabbage extract.
Overall, the findings suggest a potential in vitro drug–food interaction between cabbage extract and paracetamol, which may influence the drug’s dissolution and subsequent bioavailability. While the interaction observed is preliminary and limited to in vitro conditions, it highlights the importance of further in vivo pharmacokinetic studies to establish clinical relevance and evaluate whether cabbage consumption could impact the therapeutic efficacy of paracetamol.
Drug–food interactions can profoundly influence the pharmacokinetics and pharmacodynamics of orally administered medicines. Such interactions may alter the absorption, metabolism, or bioavailability of drugs, ultimately affecting therapeutic outcomes. Paracetamol (acetaminophen) is one of the most widely used analgesics and antipyretic drugs, with well-characterized pharmacokinetics and rapid oral absorption. Because of its predictable dissolution profile, it serves as a suitable model for evaluating potential drug–food interactions [1].
Cabbage (Brassica oleracea) is a staple dietary vegetable consumed worldwide. It is particularly rich in phytochemicals such as glucosinolates, flavonoids, and polyphenols. These compounds are known to influence metabolic enzymes and may interact with pharmaceutical compounds either chemically in solution or biologically after ingestion. Given this background, the hypothesis of this study was that cabbage extract could interfere with either the dissolution process or the UV detection of paracetamol, thereby potentially affecting its bioavailability [2].
Aim
The present study was undertaken to evaluate the in vitro drug–food interaction between paracetamol and cabbage extract using UV spectrophotometric methods and dissolution studies, modeled with a one-compartment pharmacokinetic approach.
Objectives
To assess whether cabbage extract alters the dissolution kinetics of paracetamol.
To determine whether the presence of cabbage extract interferes with UV absorbance readings of paracetamol during dissolution studies.
To model dissolution data using a one-compartment pharmacokinetic approach and compare kinetic parameters such as AUC, clearance, and half-life in the presence and absence of cabbage extract.
Study design
In this in-vitro comparative dissolution study, the potential interaction between paracetamol and cabbage extract was meticulously investigated under conditions simulating intestinal pH. The study was set up with two distinct experimental groups: a control group, where paracetamol tablets were dissolved in phosphate buffer (pH 5.8), and a test group, where the same dissolution medium contained aqueous cabbage extract [3,4].
Materials
P-500 tablet.
Phosphate buffer pH 5.8 (simulate GI pH).
Dissolution apparatus basket (USP Apparatus 1).
Method
Group-A (standard)
A standard drug, paracetamol (P-500) dropped into the dissolution basket containing buffer at pH 5.8 as per IP. Then the buffer was subjected to a dissolution process for a revolution of 30 rpm maintained at 37 ± 0.5 °C, in which paracetamol was dropped then the paddle attached continued rotating to proceed the dissolution process. The samples of the drug were withdrawn at 0, 15, 30, 45, 60, and 75 minutes, and absorbance was measured at 249 nm via UV spectrometry. The concentration vs. time graph was obtained at λmax 249 nm [5].
Group-B (test)
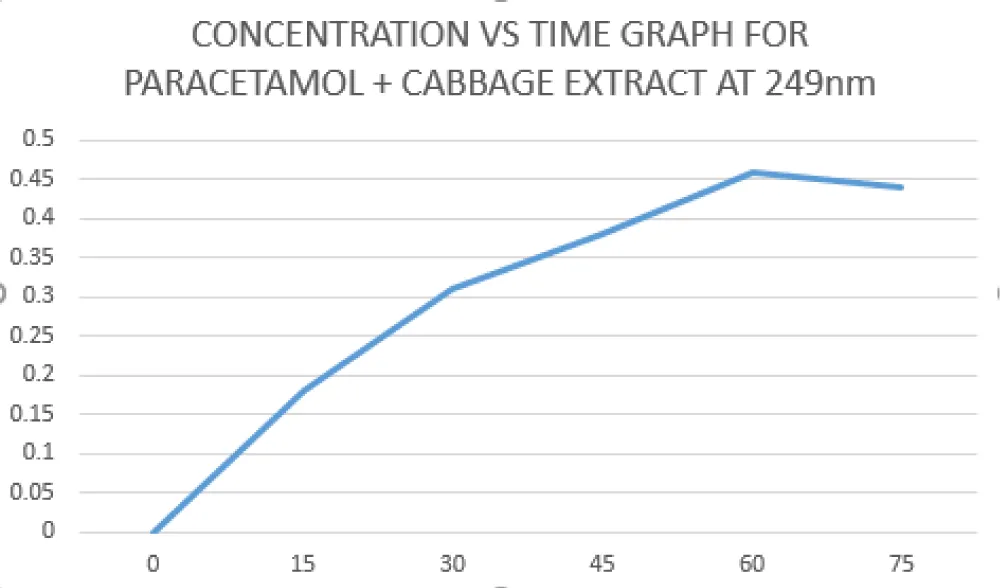
Cabbage was cut into small pieces and ground with little water, which was squeezed and filtered with a cloth, and the extract at a concentration of 1000mg/ml was obtained. The extract was poured into the phosphate buffer pH 5.8, along with this mixture, the drug (P-500) was dropped, and again the process was continued as mentioned in Group A. The graph of non-linearity was obtained when compared with the Group-A graph [6].
The number of replicates performed is 2. To analyze the dissolution profiles quantitatively, the cumulative percent drug release was computed at each time point. Additionally, data were fitted to a one-compartment pharmacokinetic model to estimate parameters such as area under the curve (AUC), apparent clearance, and half-life, providing a more pharmacological perspective on dissolution trends [7].
Control group
Paracetamol dissolution displayed a consistent, linear increase over time, reflecting expected behaviour under standard conditions.
Test group
In contrast, the cabbage extract group showed a non-linear dissolution profile. Although cumulative release percentages remained within analytical limits (absorbance ≤ 0.8), the obtained R2 value was 0.99.
The graph was obtained via UV spectroscopy absorbance measurement from withdrawing samples from both groups.
Pharmacokinetic modelling revealed two key differences in the cabbage extract samples: a reduced AUC, indicating less overall detectable drug release over time, and an increased apparent clearance, which indicates an increased elimination rate, suggesting more rapid disappearance of paracetamol from solution or reduced detectability.
These findings echo observations from literature that food substances can interfere with drug dissolution through mechanisms such as viscosity alteration, spectrophotometric overlap, or complex formation, variable gastric emptying rates depending on caloric content of meals affect paracetamol absorption kinetics—underlining how environmental factors closely modify drug release and uptake [8,9] (Figures 1-3).
Figure 1: Standard graph of paracetamol at 249 nm.
Figure 2: Concentration vs Time graph for paracetamol + cabbage extract at 249nm.
Figure 3: The graph represents the comparison of linearity between two groups (ie, control and test).
Both the groups absorbance and concentration values are calculated as per one compartment model based on that Cmax, Tmax, Ke ( elimination rate constant), Tmax, absorption rate constant (Ka), half life, AUC (area under curve), apparent clearance (CL/F), apparent volume of distribution (Vd/F) parameters were calculated and compared as per that the results are intrepreted [8,9] (Table 1).
| Table 1: Comparison of one-compartment pharmacokinetic (PK) parameters of both groups and their inferences. | |
| One Compartment PK Parameters | Inference |
| Ke ( elimination rate constant) | Increased elimination rate (indicates faster elimination of the drug) |
| Absorption rate constant (Ka) | Slightly increased absorption rate |
| Cmax | Reduced peak concentration when taken with food. |
| Tmax | Faster time to reach peak concentration. |
| Half-life (t1/2) | Half-life is longer slow elimination phase |
| AUC | Decreased in AUC |
| Apparent volume of distribution | Increased apparent volume of distribution change in the distribution pattern of the drug. |
| Apparent clearance | Increased elimination rate. |
By comparing the one compartment PK parameters of both groups the elimination rate constant (Ke) showed increase in elimination rate, absorption rate constant (Ka) showed slightly increase in absorption rate, Cmax showed decrease in peak concentration when taken with food, tmax showed faster time to reach peak concentration, half life (t1/2) showed longer slow elimination phase, AUC showed decrease in AUC, apparent volume of distribution showed increase in apparent volume of distribution, apparent clearance showed increase in elimination rate.
The study successfully demonstrates that cabbage extract can modify the in vitro dissolution dynamics of paracetamol, both through apparent reduction in drug exposure (AUC) and increased clearance metrics. These disruptions likely result from phytochemical-mediated alterations in solubility, medium properties, or spectral interference during quantification [2].
Future scope
These in-vitro studies should be correlated with in-vivo studies to confirm the interaction and should also assess any real scenario of cabbage and paracetamol interactions in patients [8].
- Prescott LF. Paracetamol: past, present, and future. Am J Ther. 2000;7(2):143–7. Available from: https://pubmed.ncbi.nlm.nih.gov/11319582/
- Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36(5):377–83. Available from: https://doi.org/10.1016/j.ctrv.2010.01.002
- Kar AK, Kar B. in-vitro comparative dissolution study of commercially available paracetamol tablets. J Drug Deliv Ther. 2020;10(1):18–23. Available from: https://jddtonline.info/index.php/jddt/article/view/3817
- Sohel MD, Rahman MM, Hossain MK, Taz S, Uddin MJ, Akhter S, Rahman MS. in-vitro dissolution interference study in the presence of paracetamol tablets with freshly prepared mango juices on simulated gastrointestinal digestion. J Pharm Nutr Sci [Internet]. 2020;10(4):148–54. Available from: https://set-publisher.com/index.php/jpans/article/view/2068
- Tarawneh OA. in-vitro characterization and evaluation of commercialized paracetamol (500 mg) immediate-release tablets using USP type II apparatus in phosphate buffer pH 5.8 and UV spectroscopy at 243 nm. Int J Pharm Sci Res. 2019;10(5).
- Li J, Wang X, Chen Y, Zhao L, Zhang Z. Dissolution and pharmacokinetic studies of paracetamol-4,4′-bipyridine cocrystals obtained using four methods. Crystals. 2025;15(1):70.
- Grassi M, Voinovich D, Grabnar I, Filipović-Grčić J. Preparation and in-vitro/in-vivo characterization of a melt-pelletized paracetamol/stearic acid sustained-release delivery system. Eur J Pharm Sci. 2004;23(5):439–46.
- Yamasaki Y, Fujita KI, Okuyama H, Izumi Y. Effects of kale ingestion on the pharmacokinetics of acetaminophen in rats. Yakugaku Zasshi. 2011;132(6):357–62. Available from: https://doi.org/10.2220/biomedres.32.357
- Pantuck EJ, Hsiao KC, Lillien L, Conney AH, Kappas A. Stimulatory effect of Brussels sprouts and cabbage on human drug metabolism. Clin Pharmacol Ther. 1979;25(1):88–95. Available from: https://doi.org/10.1002/cpt197925188