More Information
Submitted: September 10, 2025 | Approved: September 30, 2025 | Published: Ocotober 01, 2025
How to cite this article: AbuBaha M, AbuBaha B, Salameh H, Afana A, Abseh I, Taha HM. When Prophylaxis Turns Pathologic: A Case of LMWH-Induced Necrosis with Secondary Cellulitis. Arch Case Rep. 2025; 9(10): 307-309. Available from:
https://dx.doi.org/10.29328/journal.acr.1001164
DOI: 10.29328/journal.acr.1001164
Copyright license: © 2025 AbuBaha M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Low-Molecular-Weight Heparin (LMWH); Skin necrosis; Delayed-type hypersensitivity; Postpartum complications; Enoxaparin adverse reaction
When Prophylaxis Turns Pathologic: A Case of LMWH-Induced Necrosis with Secondary Cellulitis
Mohammed AbuBaha1* , Bara AbuBaha1, Hossam Salameh1, Asem Afana1, Ibrahim Abseh1 and Hatem M Taha2
, Bara AbuBaha1, Hossam Salameh1, Asem Afana1, Ibrahim Abseh1 and Hatem M Taha2
1Department of Medicine, An-Najah National University, Nablus, Palestine
2Head of Medical Departments, Palestine Medical Complex, Ramallah, Palestine
*Address for Correspondence: Mohammed AbuBaha, MD, Department of Medicine, An-Najah National University, Nablus, Palestine, Email: [email protected]
Introduction and importance: Low-molecular-weight heparins (LMWHs) are widely used for thromboprophylaxis during pregnancy and postpartum due to their favorable safety profile. However, rare immune-mediated adverse events, such as delayed-type hypersensitivity reactions leading to skin necrosis, may occur and can be complicated by secondary infections. Awareness of this rare complication is important for timely diagnosis and management.
Case presentation: We report the case of a 40-year-old postpartum woman who developed painful necrotic skin lesions one week after cesarean delivery while receiving prophylactic enoxaparin. Lesions appeared at previous injection sites on the abdomen and left arm, with associated erythema and tenderness. Laboratory findings showed leukocytosis, elevated CRP, and ESR, with normal platelet counts, excluding heparin-induced thrombocytopenia. The clinical features were consistent with LMWH-induced delayed-type hypersensitivity complicated by cellulitis. Enoxaparin was discontinued, and the patient was treated with apixaban, intravenous antibiotics, daily wound care, and partial surgical debridement. She improved and was discharged in stable condition.
Clinical discussion: Although LMWHs are considered safe, clinicians should recognize that delayed-type hypersensitivity reactions may mimic infection or thrombotic conditions, complicating diagnosis. Necrotic skin lesions typically appear 5–14 days after LMWH initiation, reflecting T-cell-mediated vascular injury. Early recognition and discontinuation of LMWH, alongside alternative anticoagulation and infection management, are crucial to prevent morbidity. Literature indicates that such cases remain extremely rare, particularly in postpartum patients, underscoring the need for heightened clinical vigilance.
Conclusion: LMWH-induced skin necrosis with secondary cellulitis is a rare but serious complication that requires prompt recognition and management. Clinicians should maintain a high index of suspicion in patients presenting with necrotic lesions at injection sites, especially in the postpartum setting, to ensure timely intervention and favorable outcomes.
Delayed-type hypersensitivity (DTH) is defined as a reaction to low-molecular-weight heparin (LMWH), and it is characterized by necrotic skin lesions, representing a rare but one of the most important side effects of heparin treatment [1]. Its immune-mediated reaction usually occurs 5–14 days after initiation of LMWH therapy and is manifested as painful, erythematous plaques that progress and develop to necrosis at injection sites [1]. The pathogenesis is a type IV hypersensitivity reaction where an inflammatory process mediated by T-cells leads to vascular damage and issue necrosis [2].
LMWHs are usually used for thromboprophylaxis in medical and surgical settings, such as during pregnancy, this is because it’s evidenced that it’s generally considered safe [3]. Their potential to trigger severe cutaneous reactions remains an underrecognized and underreported complication [3]. LMWH-induced skin necrosis is a very rare complication with an incidence rate of less than 0.1%, making it an uncommon phenomenon that presents with diagnostic dilemmas [4]. Obesity, female sex, and prolonged exposure to heparin are considered risk factors of LMWH-induced skin necrosis, though the exact mechanisms predisposing certain patients to this reaction remain unclear [2].
Histopathological findings usually include lymphocytic infiltrates, endothelial cell swelling, and microthrombosis. This feature distinguishes this condition from heparin-induced thrombocytopenia (HIT) and other thrombotic phenomena [5]. Management requires immediate discontinuation of the offending agent, alternate anticoagulants (such as fondaparinux or direct oral anticoagulants) must be used, and in some extreme situations, immunosuppressive therapy must be considered [5].
Because LMWH is generally used as thromboprophylaxis during pregnancy and because delayed diagnosis may lead to significant morbidity, this complication deserves particular attention in obstetric populations [6]. More research is required to identify risk factors, optimal diagnostic criteria, and treatment strategies for this severe cutaneous adverse reaction.
A 40-year-old woman (G3P1) presented to the emergency department with painful, dark skin lesions that developed over previous enoxaparin injection sites approximately one week after undergoing an elective cesarean section at 37 weeks of gestation for suspected fetal distress. Initially, her postpartum course was uncomplicated, and she was discharged on oral antibiotics and ibuprofen.
The patient had been receiving prophylactic enoxaparin (60 mg daily) throughout her pregnancy due to advanced maternal age and a history of two early miscarriages. She had no known thrombophilia or autoimmune conditions.
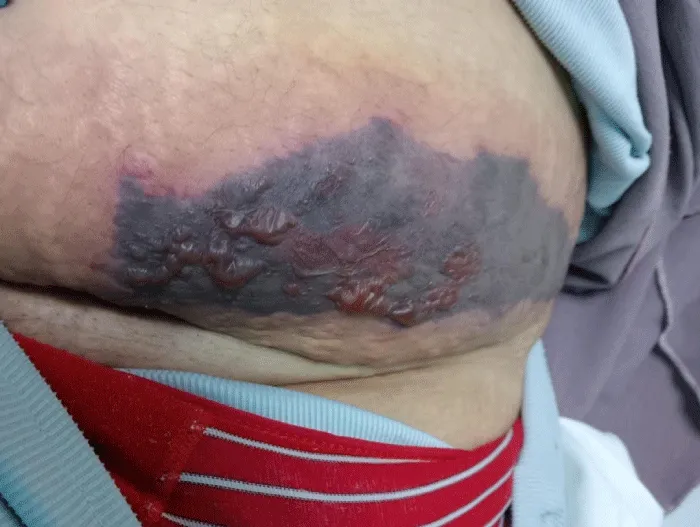
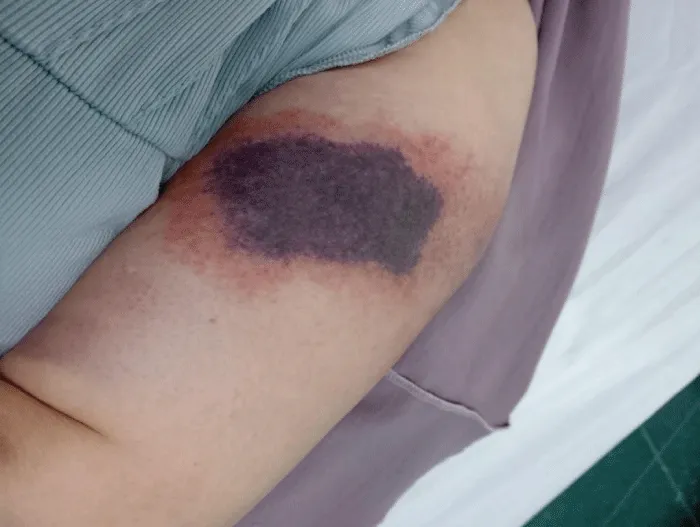
On physical examination, the patient was hemodynamically stable and afebrile. Two well-demarcated necrotic plaques were noted: one over the lower abdomen, featuring hemorrhagic bullae, and another on the left arm, presenting as a dark necrotic patch with surrounding erythema and tenderness, as seen in Figures 1,2.
Figure 1: Necrotic plaque with hemorrhagic bullae over the lower abdominal enoxaparin injection site.
Figure 2: Dark necrotic patch with erythema on the upper left arm at another injection site.
Laboratory findings revealed elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), along with mild leukocytosis. Platelet count and coagulation parameters were within normal limits. This excluded Heparin-induced thrombocytopenia (HIT).
The clinical picture was consistent with delayed-type hypersensitivity and immune-mediated skin necrosis due to low molecular weight heparin (LMWH), which was further complicated by localized cellulitis.
Management included immediate discontinuation of enoxaparin and initiation of apixaban for ongoing anticoagulation. The patient received intravenous antibiotics and daily wound care. Partial surgical debridement was performed after one week. Clinical improvement was noted, and the patient was subsequently discharged on oral antibiotics and apixaban. She was advised to avoid non-steroidal anti-inflammatory drugs (NSAIDs) and scheduled for outpatient follow-up.
The widely used Low-molecular-weight heparin (LMWH), Enoxaparin, is considered a safe option during pregnancy and postpartum for thromboprophylaxis [7]. Although rarely emerging, a clinically significant immune-mediated complication, including skin necrosis, could occur and may mimic infectious or thrombotic conditions, posing a challenge in diagnosis and management during the peripartum period [8].
As the recent literature shows, approximately 7.5% of patients on LMWH may experience a cutaneous reaction, with necrosis being distinctly rarely occurring [8,9]. The mechanisms thought to be involved in LMWH-induced skin necrosis are type IV hypersensitivity and vasculitis. To highlight the dermatological toxicities of enoxaparin, a case series from India has described enoxaparin-induced panniculitis amongst pregnant patients [10]. As well as a case report in 2022 demonstrated the rarity and the severity of enoxaparin-induced necrosis, as they noted less than 30 documented cases [11].
In our case, a 40-year-old female, 7 days after delivery, has developed necrotic skin lesions at the injection sites of enoxaparin. Exclusion of differential diagnoses was accomplished through clinical assessment, consideration of drug exposure, and laboratory testing. Heparin-induced thrombocytopenia (HIT) was ruled out by the normal platelet counts; however, elevated CRP, ESR, and WBCs increased the suspicion of localized inflammation and possible infection. She was clinically stable with no history of autoimmune diseases or thrombophilia. Her temporal pattern and presentation support a type IV hypersensitivity reaction complicated by cellulitis. According to literature, a timing of 1 to 2 weeks post-initiation for lesion development is characteristic of T-cell-mediated response [8,11].
In addition to local wound care and surgical debridement as needed, Recent literature recommendations regarding LMWH-induced skin necrosis include immediate cessation of LMWH with the switching to a non-heparin anticoagulant, and if infection was suspected, an antibiotic should be initiated [11,12]. Our patient’s course was stable and aligned with current recommendations; a broad-spectrum antibiotic course was initiated in addition to apixaban, silver sulfadiazine dressings, and surgical debridement.
A delayed cell-mediated LMWH-induced skin necrosis complicated by secondary cellulitis is a rarely occurring complication of LMWH therapy. Our patient’s recovery after a suitable management plan emphasizes the seriousness of this condition and how crucial it is for physicians to keep a close eye on patients on LMWH therapy to be able to act appropriately when unusual skin changes arise, especially during the postpartum phase, and to help raise awareness and support earlier diagnosis and better outcomes in similar situations.
Ethical considerations
All procedures performed in this report involving human participants were in accordance with the ethical standards of the institutional, national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
According to the institutional regulations, no ethical approval was required to publish any information in this case report.
Ethics statement and consent to publication
The authors obtained verbal and written informed consent from the patient regarding this case and any accompanying images. A copy of the written consent is available for review by the Editor in Chief of this journal on request.
Data availability: The data is not available to the public due to privacy concerns.
- Schindewolf M, Gobst C, Kroll H, Recke A, Louwen F, Wolter M. High incidence of heparin-induced allergic delayed-type hypersensitivity reactions in pregnancy. J Allergy Clin Immunol. 2013;132(1):131–9. Available from: https://doi.org/10.1016/j.jaci.2013.02.047
- Schindewolf M, Schwaner S, Wolter M, Kroll H, Recke A, Kaufmann R. Incidence and causes of heparin-induced skin lesions. CMAJ. 2009;181(8):477. Available from: https://doi.org/10.1503/cmaj.081729
- ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol. 2018;132(1):e1–17. Available from: https://doi.org/10.1097/aog.0000000000002706
- Handschin AE, Trentz O, Kock HJ, Wanner GA. Low molecular weight heparin-induced skin necrosis - A systematic review. Langenbecks Arch Surg. 2005;390(3):249–54. Available from: https://link.springer.com/article/10.1007/s00423-004-0522-7
- Bertrand PM, Perbet S, Sapin AF, Salavert M, Constantin JM, Elalamy I, et al. Heparin-induced skin necrosis: HIT-2 without thrombocytopenia. Intensive Care Med. 2011;37(1):172–3. Available from: https://doi.org/10.1007/s00134-010-2027-x
- Andronache R, Bi WG, Wei SQ. 886: Heparin and pregnancy outcomes: A systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222(1):S552–3. Available from: https://doi.org/10.1016/j.jogoh.2020.101974
- Pérez DL, Peña-Romero AG, Díaz-González JM, Domínguez-Cherit J. Nadroparin-induced skin necrosis: clinical manifestation of HIT-2 even in the absence of thrombocytopaenia. BMJ Case Rep. Available from: https://doi.org/10.1136/bcr-2016-215288
- Warkentin TE. Skin necrosis and other serious skin reactions. In: Heparin-Induced Thrombocytopenia. 5th ed. Marcel Dekker; 2022. Available from: https://doi.org/10.1002/ccr3.5297
- Girolami A, Cosi E, Ferrari S, Lombardi AM, Girolami B. Skin lesions due to low molecular weight heparins: A review of the literature. Clin Appl Thromb Hemost. 2017;23(8):1049–54.
- Narula S. Enoxaparin-induced cutaneous panniculitis in a pregnant female. Indian J Case Rep. 2024;9(8):239–41. Available from: https://doi.org/10.32677/ijcr.v9i8.4071
- Byrne EM, Khattab A, Chen F, Bhagavatula R. Rare presentation of enoxaparin-induced skin necrosis in a postoperative patient. BMJ Case Rep. Available from: https://doi.org/10.1136/bcr-2022-249685
- Shivamurthy P, Parker SR, Tsapepas DS. Use of direct oral anticoagulants in the management of heparin-induced thrombocytopenia: A review. J Thromb Thrombolysis. 2020;50(2):325–33.