More Information
Submitted: September 19, 2025 | Approved: September 22, 2025 | Published: September 23, 2025
How to cite this article: Manga-Mbeti J, Edinga E, Ngono L, Ngassam GLL, Mbakam LS, Omgba JPA, et al. Optimizing Hepatitis B Surface Antigen Confirmation Thresholds by Chemiluminescence Microparticle Immunoassay on the Architect i1000SR. Arch Case Rep. 2025; 9(9): 292-296. Available from:
https://dx.doi.org/10.29328/journal.acr.1001162
DOI: 10.29328/journal.acr.1001162
Copyright license: © 2025 Manga-Mbeti J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hepatitis B surface antigen (HBsAg); Chemiluminescence Microparticule Immunoassay Method (CMIA); Confirmation zone; Signal-to-cutoff ratio (S/CO); Architect i1000SR
Optimizing Hepatitis B Surface Antigen Confirmation Thresholds by Chemiluminescence Microparticle Immunoassay on the Architect i1000SR
Jeanne Manga-Mbeti* , Elodie Edinga, Laure Ngono, Ghislain Landry LonmiNgassam, Laetitia Sado Mbakam, Jean Philippe Atangana Omgba, Fatimatou Ndjoko, Annie Epote, Grace Dina and Suzanne Belinga
, Elodie Edinga, Laure Ngono, Ghislain Landry LonmiNgassam, Laetitia Sado Mbakam, Jean Philippe Atangana Omgba, Fatimatou Ndjoko, Annie Epote, Grace Dina and Suzanne Belinga
Centre Pasteur of Cameroon, PO Box 1274, Yaoundé, Cameroon
*Address for Correspondence: Jeanne Manga-Mbeti, Centre Pasteur of Cameroon, PO Box 1274, Yaoundé, Cameroon, Email: [email protected]; [email protected]
Objective: To establish an optimal confirmation zone for initial hepatitis B surface antigen (HBsAg) results obtained via Chemiluminescence Microparticle Immunoassay (CMIA) on the Architect i1000SR platform, aiming to enhance diagnostic accuracy and reduce unnecessary confirmatory testing and associated costs.
Methods: A retrospective-prospective mixed study was conducted on 231 serum samples analysed at the Centre Pasteur of Cameroon between April 2018 and December 2019. Initial results obtained using the Architect HBsAg Qualitative II assay were confirmed through the Architect HBsAg Confirmatory test. Samples encompassed a broad range of signal-to-cutoff (S/CO) ratios (0.9 –> 500). Confirmation rates were analysed across intervals. Fisher’s exact test with Bonferroni correction was applied to assess statistical differences.
Results: Of the 231 samples meeting inclusion criteria, 172 (74.5%; 95% CI: 68.3–79.9) were confirmed reactive, representing 74.8% of initially reactive samples (172/230). Confirmation rates were lowest in the 1.00–1.10 interval (9.1%) and the 1.10–10.00 interval (48.8%), both significantly lower than for samples above 200 S/CO (p < 0.0001). All samples above 200 S/CO were confirmed positive. The highest unconfirmed S/CO value observed was 170.01, supporting the effectiveness of the defined confirmation range (S/CO 0.90–200).
Conclusion: The optimal confirmation zone was defined as 0.90–200 S/CO. Confirmatory testing below this threshold is essential, while values >200 can be considered definitively positive. Implementing this strategy can reduce costs, streamline workflow and turnaround times especially in resource-limited settings without compromising accuracy.
Hepatitis B virus (HBV) infection remains a significant global health challenge, affecting over 250 million individuals worldwide and resulting in approximately one million deaths annually [1]. The infection can manifest as both acute and chronic forms, with chronic hepatitis B leading to severe liver complications such as cirrhosis and hepatocellular carcinoma [2,3]. Transmission primarily occurs through perinatal exposure, close contact with infected blood or bodily fluids, sexual contact, and unsafe injection practices [4,5]. Despite the introduction and widespread deployment of effective vaccines that have substantially reduced the prevalence of HBV, many regions continue to face high endemicity. According to the World Health Organisation (WHO), as of 2022, only about 13% of persons living with HBV were aware of their infection, and merely 3% received treatment, underscoring the ongoing public health burden [1].
Early and accurate diagnosis of HBV infection is vital for initiating appropriate clinical management, reducing transmission, and implementing effective public health interventions. The detection of hepatitis B surface antigen (HBsAg) remains the primary diagnostic marker for active and chronic infection [6,7]. Modern serological testing methods, especially Chemiluminescence Microparticle Immunoassay Methods (CMIA), offer high sensitivity and specificity; however, they can also produce false-positive or equivocal results, particularly when values are near the assay’s cutoff thresholds [8,9]. Such results necessitate confirmatory testing to distinguish true infections from false reactivities, ensuring optimal use of healthcare resources and improving diagnostic confidence.
Current guidelines emphasise the importance of confirmatory testing for initial reactive HBsAg results, particularly those close to the positive cutoff, to prevent misdiagnosis and unnecessary treatments [10-12]. Nonetheless, the criteria and thresholds for confirmation vary across settings, and overly restrictive or broad strategies may lead to either missed diagnoses or unnecessary follow-up testing, thereby impacting costs and clinical decision-making [13]. Optimizing the confirmation zone—the specific range of signal-to-cutoff (S/CO) ratios that reliably predicts true positivity—could streamline laboratory workflows, reduce costs, and improve diagnostic accuracy.
This study aims to define the optimal confirmatory zone of initial HBsAg results obtained by CMIA on the Architect i1000SR platform. Establishing such a threshold is expected to enhance diagnostic accuracy while reducing costs and turnaround time, thereby supporting public health programs and improving patient management, particularly in high-endemic settings and low- and middle-income countries.
Study design and setting
This study was conducted at the Centre Pasteur of Cameroon and employed a mixed retrospective and prospective approach from April 2018 to December 2019. The primary objective was to determine the optimal S/CO confirmation zone for HBsAg testing utilizing the Architect i1000SR platform (Abbott Diagnostics).
Study population and sample collection
Participants included individuals undergoing HBV screening. Samples with initial S/CO ratios between 0.9 and 500 were included. Samples exceeding 500 S/CO were considered definitively positive and used as positive controls for performance assessment. The samples were stratified into three categories based on initial S/CO: negative (< 0.90), gray zone (0.90–1.10), and positive (≥ 1.10).
Laboratory procedures
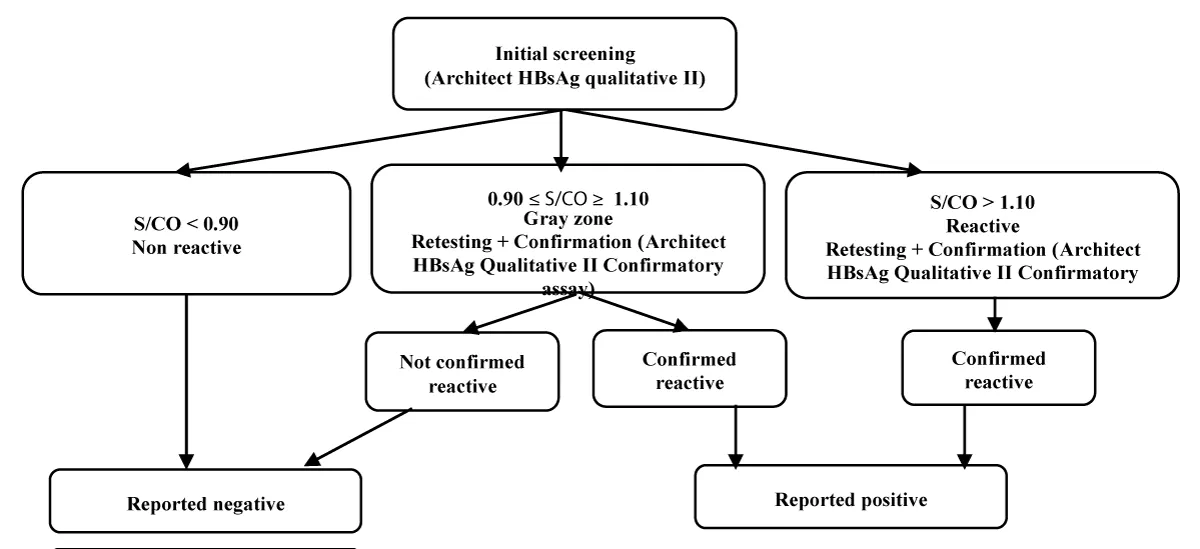
All assays were performed following the manufacturers’ instructions within a certified laboratory adhering to quality standards. Initial screening employed the Architect HBsAg Qualitative II assay. Reactive samples were confirmed using the Architect HBsAg Qualitative II Confirmatory assay, which employs an antibody-mediated neutralization principle (Figure 1). Confirmation was deemed positive if neutralization was successful; otherwise, the results were considered false positives. Data collection and record management adhered to strict quality protocols, with systematic documentation in Microsoft Excel 2010 to ensure traceability.
Figure 1: Laboratory workflow for HBsAg testing on the Architect i1000SR. Initial screening results are classified according to S/CO thresholds. Samples in the grey zone or reactive range undergo confirmatory testing, which differentiates true positives from false reactive results.
Data analysis and determination of the confirmation zone
Samples were stratified into 9 S/CO intervals, and confirmation rates were calculated as the proportion of initially reactive samples that tested reactive upon confirmation, with 95% confidence intervals (CI). True positives (TP), false positives (FP), true negatives (TN), and false negatives (FN) were identified based on confirmatory results.
Fisher’s exact test with the Bonferroni correction assessed differences from the reference group (threshold interval above which all samples show confirmed reactive ratios), with the adjusted p - value < 0.05/9 considered significant. The confirmation zone was defined as the S/CO interval where confirmatory testing remained necessary to ensure accurate diagnosis. Sensitivity analyses employed uncorrected p - values to evaluate the robustness of findings.
Ethical considerations
Patient confidentiality was maintained by anonymization. The study was approved by the institutional review board.
Sample analysis and classification results
A total of 231 samples were analyzed, including 224 with S/CO ratios between 0.90 and 500, and 7 with S/CO > 500 used as positive controls. Initial screening classified 230 samples (99.57%) as reactive and 1 (0.43%) as non-reactive. According to the laboratory algorithm, 12 samples (5.19%) fell within the equivocal zone, while 219 (94.81%; 95% CI: 92.9–96.4) were considered reactive. The distribution of S/CO values across predefined intervals and classifications is presented in Table 1.
| Table 1: Distribution of samples and classification by S/CO intervals. | ||||
| S/CO interval | Number of Abbott Samples | Laboratory algorithm interpretation | Frequency (%) | |
| [0.90–1.00] | 1 | Non-reactive | Grey zone | 0.43% |
| [1.00–1.10] | 11 | Reactive | Grey zone | 4.76% |
| [1.10–10.00] | 80 | Reactive | Reactive | 34.63% |
| ]10.00–50.00] | 40 | Reactive | Reactive | 17.32% |
| [50.00–100.00] | 17 | Reactive | Reactive | 7.36% |
| [100.00–150.00] | 18 | Reactive | Reactive | 7.79% |
| [150.00–200.00] | 10 | Reactive | Reactive | 4.33% |
| [200.00–500.00] | 47 | Reactive | Reactive | 20.35% |
| >500 | 7 | Reactive | Reactive | 3.03% |
| Total | 231 | - | - | 100% |
Confirmation outcomes and S/CO stratification
Out of 231 samples, 172 (74.5%; 95% CI: 68.3–79.9) were confirmed reactive. The difference with the reference group (> 200 S/CO, all confirmed positive) was highly significant (p < 0.0001). Non-confirmed results mainly occurred in low S/CO ranges below 500 S/CO.
Stratified analysis by S/CO intervals (Table 2) showed significant variation in confirmation rates. The lowest was in the [1.00–1.10] interval (9.1%), followed by [1.10–10.00] (48.8%), both significantly lower than the reference group (> 200 S/CO, 100% confirmed; corrected p < 0.0001). In contrast, intervals above 50 S/CO showed near-perfect confirmation rates (≥ 80%–100%), with no significant differences compared with the reference after Bonferroni correction. The 150–200 interval had an intermediate rate (80.0%; corrected p = 0.2173), suggesting caution in interpretation due to potential false positives.
| Table 2: Testperformance based on the S/CO interval and confirmation rate of HBsAgresults. | ||||||||
| S/CO Interval | Number | TP | FP | TN | FN | ConfirmationRate % (95% CI) | p-value | p -value (Corrected) |
| [0.90–1.00] | 1 | 0 | 0 | 1 | 0 | 0.0 (0.0 – 97.5) | 1 | 1 |
| [1.00–1.10] | 11 | 1 | 10 | 0 | 0 | 9.1 (0.2 – 41.3) | <0.0001 | <0.0001 |
| [1.10–10.00] | 80 | 39 | 41 | 0 | 0 | 48.8 (37.4 – 60.2) | <0.0001 | <0.0001 |
| [10.00–50.00] | 40 | 35 | 5 | 0 | 0 | 87.5 (73.2 – 95.8) | 0.012 | 0.1079 |
| [50.00–100.00] | 17 | 17 | 0 | 0 | 0 | 100.0 (80.5 – 100.0) | 1 | 1 |
| [100.00–150.00] | 18 | 18 | 0 | 0 | 0 | 100.0 (81.5 – 100.0) | 1 | 1 |
| [150.00–200.00] | 10 | 8 | 2 | 0 | 0 | 80.0 (44.4 – 97.5) | 0.022 | 0.2173 |
| [200.00–500.00] | 47 | 47 | 0 | 0 | 0 | 100.0 (92.4 – 100.0) | 1 | 1 |
| >500 | 7 | 7 | 0 | 0 | 0 | 100.0 (56.6 – 100.0) | 1 | 1 |
| Total | 231 | 172 | 58 | 1 | 0 | 74.5(68.3 – 79.9) | <0.0001 | <0.0001 |
| Legend: Tp = true positives (confirmed reactive); Fp = false positives (initiallyreactive but confirmed non-reactive); TN = true negatives; FN = falsenegatives. *Confirmation rate = TP / N with 95%confidence intervals (CI). | ||||||||
Uncorrected Fisher’s exact test for the [10.00–50.00] interval approached significance (p = 0.049), indicating a possible difference, though this was not maintained after correction. This underscores the importance of the correction in establishing robust confirmation thresholds.
p - values were calculated using Fisher’s exact test, with each S/CO interval compared against the reference group (>200 S/CO, all confirmed reactive). Multiple testing was adjusted with the Bonferroni method; the global corrected significance threshold was set at p < 0.005.
Defining the confirmation zone
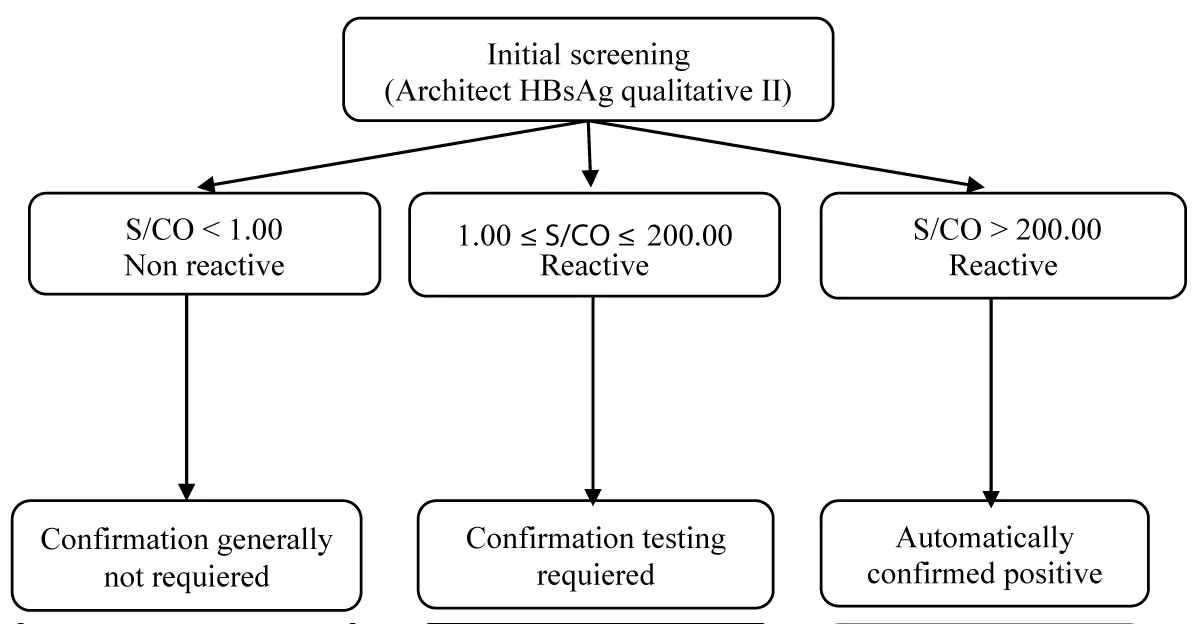
The optimal confirmation zone was defined as S/CO values between 1.00 and 200. Confirmatory testing is critical in this range, particularly at the lower end, where false reactivity was common. Samples in the 1.00–1.10 interval had a very low confirmation rate (9.1%), highlighting the unreliability of results near the positivity cutoff. In contrast, all samples with S/CO > 200 were confirmed positive (100%), indicating that confirmation can be safely omitted beyond this threshold without loss of diagnostic accuracy. This stratification provides a practical and cost-effective framework to improve diagnostic workflows and resource allocation, consistent with best practices in laboratory quality management (Figure 2).
Figure 2: Proposed confirmation strategy based on S/CO intervals.
Hepatitis B surface antigen (HBsAg) is a key marker for diagnosing active hepatitis B virus (HBV) infection, detectable in both acute and chronic phases. In our study of 231 samples, 99.57% (230/231) were initially reactive, reflecting the high sensitivity of the current CMIA platform.
However, 5.19% (12/231) of results fell within the laboratory-defined “gray zone,” requiring additional confirmation to ensure diagnostic reliability.
Overall, 74.78% (172/230) of initially reactive samples were confirmed as true positives, a rate higher than that reported by Shao, et al. [14], who observed 59% on the Roche Cobas e601 platform, particularly in the low S/CO range (0.9–10.0). When our analysis was restricted to this same interval (0.90–10.0 S/CO), the confirmation rate decreased to 44.56% (1 confirmed negative and 40 confirmed positives among 92 samples). A similar trend was reported by Kim, et al. [15], who evaluated 133 samples using the Elecsys HBsAg II assay and observed a 70.7% confirmation rate, with positivity defined by neutralization outcomes. More recently, Madiyal, et al. [12] highlighted the added value of confirmatory testing on the Roche Cobas platforms, showing an 87.71% reduction in equivocal results with the Elecsys HBsAg II kit (mean COI 12 ± 10.9). Collectively, these findings emphasize how assay technology, sample characteristics, and grayzone definitions can markedly influence confirmation outcomes, reinforcing the need for clear and standardized confirmation thresholds.
Stratified analysis revealed that false positives predominated at low S/CO values. Confirmation was extremely poor in the [1.00–1.10] interval (9.1%) and remained low in the [1.10–10.00] range (48.8%). These findings align with Shao, et al. [14] and Lee, et al. [16], who reported that more than 40% of weakly reactive results (1.0–2.0 S/CO) were false positives and would have been misclassified without neutralization testing. Similarly, Purnamawaty, et al. [11] identified 1.08 COI as the optimal cutoff requiring confirmation, achieving 89.7% specificity and 64.7% sensitivity. This threshold closely matches the zone in our study where false positives were most common, reinforcing the need for confirmatory testing in this range. Pasaribu, et al. [17] further supported these findings, showing that all unconfirmed results had S/CO values < 10, with ROC analysis defining 0.98–9.32 as the optimal confirmation zone (AUC = 83.3%, p < 0.001). Together, these data strongly support mandatory confirmation for results below 10 S/CO and provide robust evidence for a broader “confirmation zone” extending up to 200 S/CO.
Importantly, our results also showed that all samples with S/CO ≥200 were confirmed positive, suggesting that above this threshold, confirmatory testing may safely be omitted. This is consistent with O’Brien [18], who proposed that only weakly reactive samples require confirmation, and with other studies demonstrating that high S/CO values reliably predict true positivity. However, the [150–200] interval deserves attention: despite its relatively high ratios, 20% of results in this range were false positives. These anomalies may reflect mutant HBsAg variants escaping neutralization or analytical interferences during screening.
Statistical analysis strengthens these findings. Using Fisher’s exact test with Bonferroni correction, only the [1.00–1.10] and [1.10–10.00] intervals showed significantly lower confirmation rates compared with the reference group (>200 S/CO), with corrected p < 0.0001. While uncorrected p - values suggested that the [10.00–50.00] interval might also differ (p = 0.049), this association disappeared after correction, confirming that only low S/CO values (< 10) truly behave differently. This conservative statistical approach reduces the risk of false-positive associations and supports defining the confirmation zone as 0.90–200 S/CO. Within this framework, values ≥ 50 S/CO behave statistically like those >200, justifying exemption from routine confirmation, while results near the cutoff require strict verification.
Limitations and implications
Several limitations must be acknowledged. Data loss from 2018 limited the completeness of sample records, and small sample sizes in some intervals restrict the generalizability of the thresholds. Moreover, as this study was conducted in a high-endemic setting (Cameroon) using the Architect i1000SR, external validation is needed across populations and platforms.
Despite these constraints, our findings have clear practical implications. Defining >200 S/CO as a definitive threshold could reduce unnecessary confirmatory tests, saving time, reagents, and costs without compromising accuracy. At the same time, mandatory confirmation for values between 1.00 and 10.00 S/CO prevents false diagnoses and ensures clinical reliability. Implementing such stratified strategies enhances diagnostic efficiency and aligns with best practices in laboratory quality management.
Key findings
- Confirmed nearly all samples with S/CO >200.
- Confirmation rates increase progressively with S/CO.
- The optimal confirmation zone is between 0.90 and 200 S/CO.
Our analysis supports a practical confirmation policy for HBsAg testing on the Architect i1000SR: mandatory confirmatory testing for S/CO 0.90–200, especially for values under 10, and acceptance of S/CO ≥200 as effectively confirmed. This strategy provides a sound compromise between diagnostic safety and operational efficiency and can help laboratories in resource-limited and high-volume settings optimize reagent use and turnaround time while maintaining high diagnostic quality.
Funding statement
This study was funded by the Centre Pasteur of Cameroon. The funding body played no role in the design, data collection, analysis, interpretation, or manuscript preparation.
The authors thank the staff of the Serology Department of the Centre Pasteur of Cameroon for their support in data collection.
Authors’ contributions
JMM conceived the study, designed the research protocol, and performed statistical analyses. E.E., L.N., G.L.N., L.S.M., J.P.A.O., and F.N. collected data and contributed to the interpretation of results. A.E., G.D., and S.B. critically reviewed the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Data availability statement
The authors declare that data supporting the findings of this study are included in the article, and that additional information is available from the corresponding author upon reasonable request. No data from other sources was used in this manuscript.
- World Health Organization. Global hepatitis report 2024: action for access in low-and middle-income countries. Geneva: World Health Organization. 2024. Available from: https://www.who.int/publications/i/item/9789240091672
- Tustumi F, Xavier das Neves R, Pereira MA, Coelho FF, Andraus W. Editorial: Liver cancer awareness month 2023: current progress and future prospects on advances in primary liver cancer investigation and treatment. Front Oncol. 2024;14:1453709. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2024.1453709/full
- Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313‑2324. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31865-8/fulltext
- Sabeena S, Ravishankar N. Horizontal modes of transmission of hepatitis B virus (HBV): a systematic review and meta-analysis. Iran J Public Health. 2022;51(10):2181‑2193. Available from: https://ijph.tums.ac.ir/index.php/ijph/article/view/29123
- Riches N, Henrion MYR, MacPherson P, Hahn C, Kachala R, Mitchell T, et al. Vertical transmission of hepatitis B virus in the WHO African region: a systematic review and meta-analysis. Lancet Glob Health. 2025;13(3):e447‑e458. Available from: https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(24)00506-0/fulltext
- Pronier C, Candotti D, Boizeau L, Bomo J, Laperche S, Thibault V. The contribution of more sensitive hepatitis B surface antigen assays to detecting and monitoring hepatitis B infection. J Clin Virol. 2020;129:104507. Available from: https://www.sciencedirect.com/science/article/pii/S1386653220301580
- Kuhns MC, Holzmayer V, McNamara AL, Sickinger E, Schultess J, Cloherty GA. Improved detection of early acute, late acute, and occult hepatitis B infections by an increased sensitivity HBsAg assay. J Clin Virol. 2019;118:41‑45. Available from: https://www.sciencedirect.com/science/article/pii/S1386653219302126
- Wongchampa P, Chaiwongkot A, Reantragoon R. Determination of initial hepatitis B surface antigen (HBsAg) cutoff index (COI) threshold for reporting HBsAg reactivity. Chulalongkorn Med J. 2024;68(2). Available from: https://he05.tci-thaijo.org/index.php/CMJ/article/view/1475
- Khadem-Ansari MH, Omrani MD, Rasmi Y, Ghavam A. Diagnostic validity of the chemiluminescent method compared to polymerase chain reaction for hepatitis B virus detection in the routine clinical diagnostic laboratory. Adv Biomed Res. 2014;3:116. Available from: https://www.advbiores.net/article.asp?issn=2277-9175;year=2014;volume=3;issue=1;spage=116;epage=116;aulast=Khadem-Ansari
- Lee J, Lee SY, Cho YG, Kim DS, Park J. Accuracy validation of the Elecsys HBsAg II Quant assay and its utility in resolving equivocal qualitative HBsAg results. Medicina (Kaunas). 2023;59(3):443. Available from: https://www.mdpi.com/1648-9144/59/3/443
- Purnamawaty S, Handayani I, Nurulita A, Bahrun U. Determination of reactive HBsAg cutoff that needs a confirmatory test. Indones J Clin Pathol Med Lab. 2018;24(3):251‑254. Available from: https://www.indonesianjournalofclinicalpathology.org/index.php/patologi/article/view/1335
- Madiyal M, Vishwanath S, Shetty S. Role of HBsAg neutralisation test in low positive and indeterminate HBsAg results by electrochemiluminescence. J Pure Appl Microbiol. 2024;18(1):549‑554. Available from: https://microbiologyjournal.org/abstract-18-1-38/
- Tiwari AK, Pabbi S, Aggarwal G, Arora D, Bhardwaj G, Setya D, et al. Application of sequential serological testing strategy for detection of hepatitis B surface antigen (HBsAg) for diagnosing HBV infection. J Virol Methods. 2019;274:113726. Available from: https://www.sciencedirect.com/science/article/pii/S0166093419301054
- Shao H, Li Y, Xu WZ, Zhou X. Increased need for testing to confirm initial weakly reactive results for hepatitis B virus surface antigen. Lab Med. 2012;43(4):15‑17. Available from: https://academic.oup.com/labmed/article/43/4/15/2504983
- Kim SK, Huh J, Jeong TD. Proposal of efficient workflows for confirmatory neutralization test for initial hepatitis B surface antigen positive samples. Clin Lab. 2019;65(10). Available from: https://www.clin-lab-publications.com/article/3121
- Lee MY, Kang SY, Lee WI, Kim MH. Need for confirmatory neutralization tests for hepatitis B surface antigen tests in populations with intermediate prevalence. Lab Med. 2021;52(5):485‑492. Available from: https://academic.oup.com/labmed/article/52/5/485/6161312
- Pasaribu MM, Wonohutomo JP, Immanuel S, Kumalawati J, Indrasari ND, Yusra Y. Cutoff value of qualitative HBsAg for confirmatory HBsAg using the chemiluminescence microparticle immunoassay method. Lab Med. 2022;53(5):475‑478. Available from: https://academic.oup.com/labmed/article/53/5/475/6593564
- O’Brien JE. Hepatitis B surface antigen: decreased need for confirmation of reactive results. Clin Chem. 2000;46(4):582. Available from: https://pubmed.ncbi.nlm.nih.gov/10759492/