More Information
Submitted: September 04, 2025 | Approved: September 16, 2025 | Published: September 17, 2025
How to cite this article: Jarrar L, Mashni A, Fuqha R, Zaid M. Expanding the Phenotype of OTUD6B-Related Disorder: Neurodevelopmental Impairment and Immune Dysregulation with Immune Thrombocytopenic Purpura: A Case Report. Arch Case Rep. 2025; 9(9): 286-291. Available from:
https://dx.doi.org/10.29328/journal.acr.1001161
DOI: 10.29328/journal.acr.1001161
Copyright license: © 2025 Jarrar L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Case report; Mutation; Immune Thrombocytopenic Purpura; OTUD6B
Expanding the Phenotype of OTUD6B-Related Disorder: Neurodevelopmental Impairment and Immune Dysregulation with Immune Thrombocytopenic Purpura: A Case Report
Lilyan Jarrar1*, Ahmad Mashni2, Raya Fuqha3 and Mahdi Zaid4
1MD, Intern Doctor, Jenin Governmental Hospital, Jenin, Palestine
2MD, Class of 2024, An-Najah National University, Faculty of Medicine and Health Sciences
3MD, Intern Doctor, Jenin Governmental Hospital, Jenin, Palestine
4MD, Pediatrician, Jenin Governmental Hospital, Jenin, Palestine
*Address for Correspondence: Lilyan Jarrar, MD, Intern Doctor, Jenin Governmental Hospital, Jenin, Palestine, Email: [email protected]
Background: OTUD6B-related disorder is a rare autosomal recessive condition primarily associated with neurodevelopmental impairment, seizures, and dysmorphic features. Its possible role in immune dysregulation has not been well documented.
Case presentation: We describe an 11-year-old Palestinian female with a homozygous OTUD6B (c.173-2A>G) mutation who presented with global developmental delay, cavovarus foot deformities, scoliosis, seizures, and recurrent infections. During hospitalization, she developed severe thrombocytopenia (platelet count 8,000/µL) with epistaxis, hematemesis, and a purpuric rash. Laboratory evaluation confirmed immune thrombocytopenic purpura (ITP). She responded to intravenous immunoglobulin (1 g/kg, total dose 18 g), methylprednisolone, and supportive care, with platelet recovery to 17,000/µL within 24 hours. Her younger sister, also carrying the same OTUD6B mutation, exhibited developmental delay and seizures but no hematologic complications.
Discussion: This case suggests a potential novel association between OTUD6B mutations and immune dysfunction, particularly ITP. The observation expands the clinical spectrum of OTUD6B-related disorders beyond neurodevelopmental impairment, raising the possibility of immune dysregulation through disrupted deubiquitination pathways and type I interferon signaling.
Conclusion: Recognition of hematologic manifestations in OTUD6B-related disorder is essential for comprehensive patient management and genetic counseling. Further research is needed to clarify the immunological role of OTUD6B and its contribution to autoimmune conditions.
Deubiquitination plays a fundamental role in various cellular processes, including protein degradation, DNA repair, and immune signaling. Deubiquitinating enzymes (DUBs) regulate immune responses by modulating key pathways involved in inflammation, antiviral defense, and immune homeostasis [1]. Disruptions in these enzymes have been implicated in autoimmune and inflammatory conditions, highlighting their critical role in maintaining immune balance. OTUD6B, a member of the OTU family of DUBs, is essential for neurodevelopment, with biallelic mutations linked to a syndrome characterized by intellectual disability, seizures, and dysmorphic features [2]. While the neurological consequences of OTUD6B mutations are well-documented, their impact on immune regulation remains largely unexplored. Given the established role of DUBs in immune regulation, mutations in OTUD6B may disrupt key immune pathways, potentially contributing to autoimmune manifestations.
Immune thrombocytopenic purpura (ITP), the most common acquired thrombocytopenia in children, is typically an acute and self-limiting autoimmune disorder. However, its occurrence in the context of a genetic syndrome such as OTUD6B-related disorder is novel and raises the possibility that OTUD6B mutations may contribute to immune dysregulation. Here, we describe a case of a child with a homozygous OTUD6B (c.173-2A>G) mutation who developed ITP. This observation suggests a possible role for OTUD6B in immune homeostasis and raises important questions about its broader immunological significance. Understanding the link between OTUD6B dysfunction and immune dysregulation may expand the known phenotype of OTUD6B-related disorders.
We present the case of an 11-year-old Palestinian female with a complex clinical presentation, including developmental delay, cavovarus foot deformities, scoliosis, and severe thrombocytopenia. She was born at term via cesarean section with a birth weight of 2600 grams and had an uneventful delivery. Her prenatal history was notable for polyhydramnios, though no detailed fetal ultrasound was performed. There was no history of maternal teratogenic exposure, and her birth was uncomplicated, with Apgar scores of 8 at both 1 and 5 minutes. A newborn hearing screening confirmed normal auditory function, and her vision was formally evaluated and found to be normal.
Early growth parameters were within normal limits for weight and length, but her head circumference remained below the 3rd percentile. By three years of age, she exhibited signs of growth delay in weight and length. At her most recent evaluation, growth measurements showed weight 10 kg (< 3rd percentile), height 80 cm (< 5th percentile), and head circumference 45 cm (< 2nd percentile), consistent with chronic growth restriction. Developmental delays became evident by six months of age, as she was unable to sit or crawl. By three years, she could walk with support, but her gait remained unsteady. At eight years, she lost the ability to walk due to progressive cavovarus foot deformities.
The patient’s first seizure occurred at 4 years of age, manifesting as generalized tonic–clonic status epilepticus, which required hospitalization and subsequent initiation of sodium valproate. Seizure control has since been maintained with regular therapy. Developmental delays were apparent from six months of age, with significant lag across motor, language, and social domains. A summary of milestones is provided in Table 1, highlighting global developmental impairment.
| Table 1: Developmental milestones, | |||
| Domain | Normal Range | Patient’sAge Achieved | Comment |
| Head control | 3–4 months | 6 months | Delayed |
| Sitting unsupported | 6–8 months | 12 months | Delayed |
| Walking | 12–15 months | 3 years (with support) | Significantly delayed; lost ambulation at 8 years due to cavovarus deformities |
| First words | 12–15 months | 24 months | Delayed |
| Social smile | 2 months | 3 months | Mildly delayed |
Her medical history includes multiple hospitalizations due to recurrent pulmonary infections, and at four years old, she experienced an episode of status epilepticus. She was subsequently placed on sodium valproate, which has effectively controlled her seizures since then. She was both breastfed and formula-fed during infancy, and currently, she consumes small, frequent meals without gastrointestinal issues like diarrhea, constipation, or vomiting. She remains in diapers and has not experienced recurrent urinary tract infections.
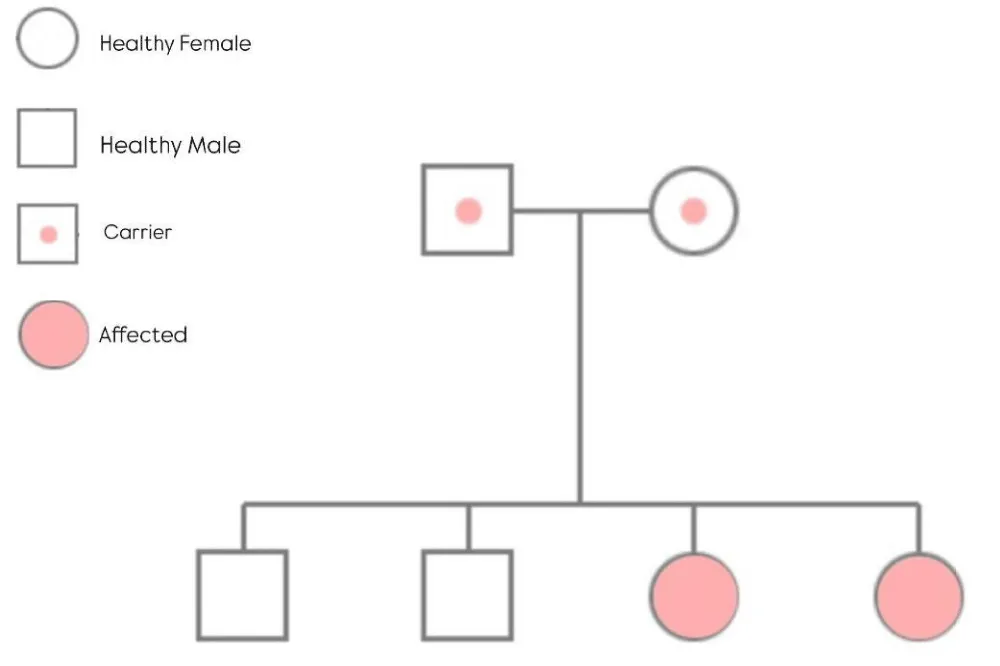
Family history includes her younger sister, who shares the same OTUD6B gene mutation and also has developmental delays, hypotonia, and seizures, which are well-managed with phenytoin. There is no relevant family history of other genetic disorders or autoimmune diseases. Additionally, Figure 1 depicts the family pedigree, providing insight into hereditary patterns associated with the condition.
Figure 1: This pedigree highlights the autosomal recessive transmission of the OTUD6B-related disorder within the family.
Examination
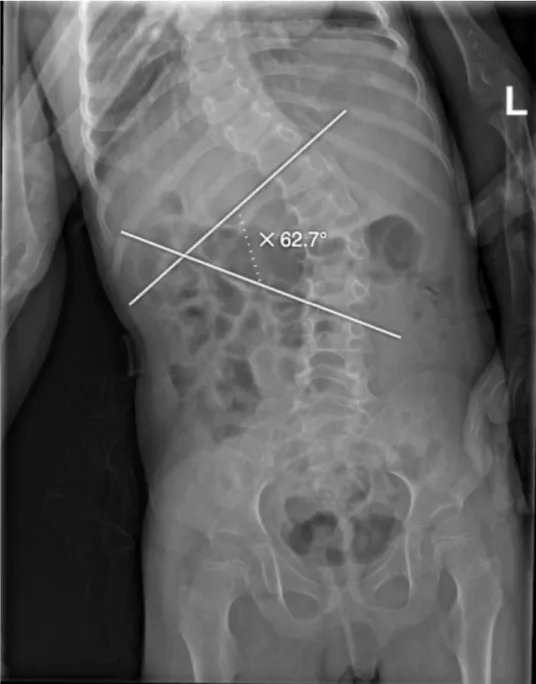
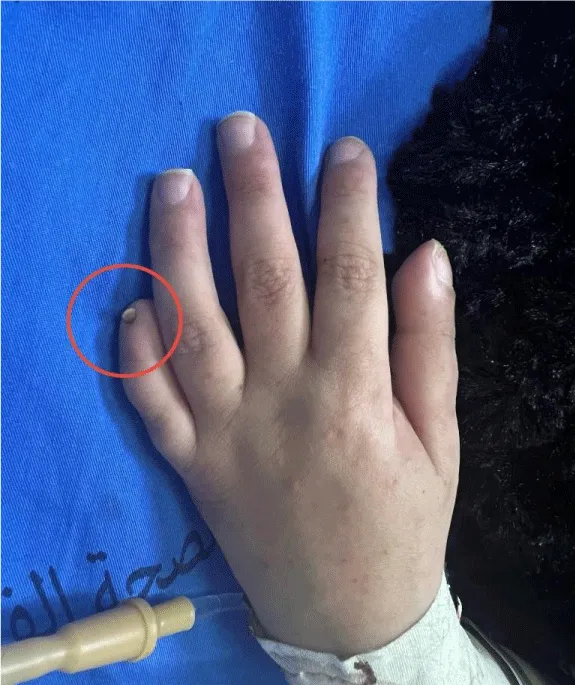
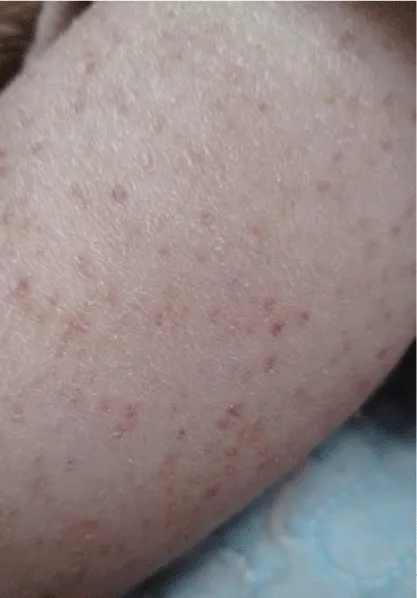
At the time of her most recent hospitalization, she presented with an episode of epistaxis, multiple episodes of hematemesis, and a purpuric rash. Physical examination revealed several dysmorphic features, including micrognathia, broad nasal tip, hypertelorism, thick eyebrows, large, low-set ears, and a short neck. Musculoskeletal examination revealed marked hypotonia in both upper and lower extremities, cavovarus feet, scoliosis with a Cobb angle of 62.7°, and hypoplastic nails, particularly in the fifth fingers (Figures 2,3). Her skin was dry with absent body hair and a purpuric rash (Figure 4).
Figure 2: Spine X-ray showing scoliosis with a Cobb angle of 62.7°, indicating a severe spinal curvature.
Figure 3: Hypoplastic nail of the fifth finger (red circle), a feature associated with OTUD6B mutation.
Figure 4: Purpuric rash with dry skin in the patient’s forearm, who presented with epistaxis and hematemesis.
Other systems, including cardiovascular, respiratory, gastrointestinal, and genitalia, were unremarkable.
Differential diagnosis
Several conditions were considered, given the patient’s clinical features. Neuromuscular disorders such as hereditary motor neuropathies or congenital myopathies were considered due to her early motor delay and progressive foot deformities. However, the absence of significant weakness, normal deep tendon reflexes, and lack of clear neuropathic or myopathic patterns made these diagnoses less likely.
Syndromic conditions like Noonan syndrome and Ehlers-Danlos syndrome were also considered due to the growth delay, dysmorphic features, and hypotonia. However, the absence of congenital cardiac defects (in Noonan syndrome) and characteristic craniofacial features (in Ehlers-Danlos syndrome) made these diagnoses unlikely.
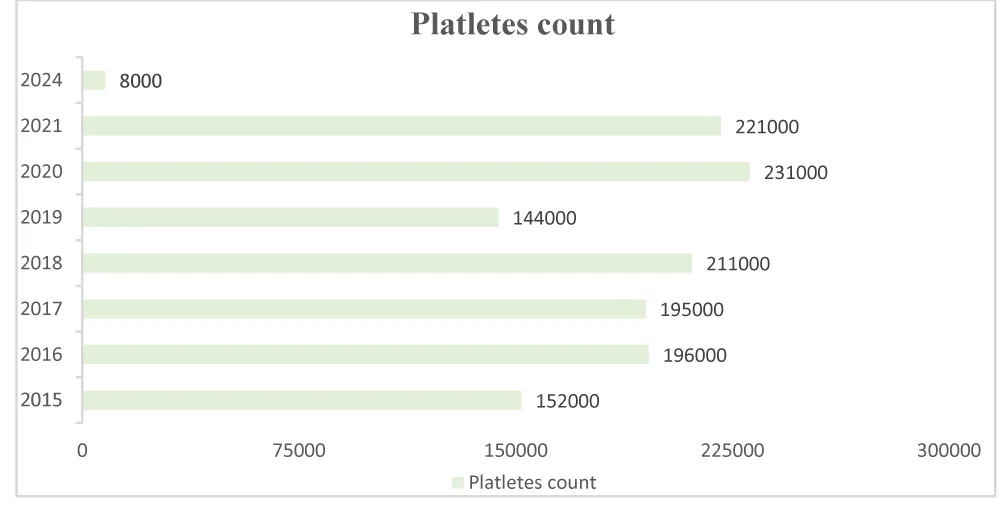
Her severe thrombocytopenia and purpuric rash raised concerns for immune thrombocytopenic purpura (ITP), inherited platelet disorders, or bone marrow failure syndromes. The acute decline in platelet count (8000/µL) in 2024, as shown in Figure 5, contrasted with her previously stable platelet levels, favors an acquired immune-mediated process (ITP) over a congenital thrombocytopenia. Her rapid response to IVIG further supported this diagnosis.
Figure 5: Patient’s platelet count trend over the years. The graph illustrates a significant drop in platelet count in 2024 (8000/µL) compared to previous years, where values remained within the normal range.
Coffin-Siris syndrome (CSS) was also considered due to the patient’s developmental delay, dysmorphic features, hypotonia, and skeletal abnormalities (e.g., cavovarus foot deformities and scoliosis). This syndrome is typically caused by mutations in genes involved in chromatin remodeling, such as ARID1B, ARID1A, SMARCB1, SMARCA4, and SS18. However, genetic testing revealed a homozygous OTUD6B mutation, leading to a different diagnosis, as this mutation is not associated with Coffin-Siris syndrome.
Investigations
Genetic testing was performed through whole exome sequencing (WES), which revealed a homozygous pathogenic mutation in the OTUD6B gene (c.173-2A>G). Both parents were found to be carriers of this mutation, though there was no known consanguinity. Despite the genetic findings, no additional syndromic features were identified in the patient.
Laboratory investigations confirmed severe thrombocytopenia, with a platelet count of 8000/µL, and a normal coagulation profile, suggesting Immune Thrombocytopenic Purpura (ITP) as the most likely diagnosis. Other routine blood tests were normal, and imaging studies, including skeletal radiographs, were consistent with the clinical findings of scoliosis and cavovarus foot deformities.
Treatment
The patient was treated with intravenous immunoglobulin (IVIG) at a dose of 1g/kg (total dose: 18g) over six hours, along with methylprednisolone (18 mg every 8 hours for two days, followed by a tapering regimen) and pantoprazole (20 mg every 12 hours). Her condition improved significantly, with her platelet count rising to 17,000/µL within 24 hours post-IVIG administration, as shown in Table 2.
| Table 2: Laboratory Results during hospitalization. | ||
| Results | Reference range | |
| Hemoglobin | 8.7 | 11.5-15.5 g/dL |
| RBCs | 4.18 | 4-5.2 M/uL |
| MCV | 70.6 | 76-80 fL |
| WBCs | 2.6 | 4.5-13.5 K/uL |
| Platelet distribution width | 17 | 10 (GSD) |
| Platelets | ||
| Day One of admission | 8000 | 150-450 K/uL |
| Day Two of admission | 6000 | |
| 24hr post IVIG | 17000 | |
| Coagulation profile | ||
| PT | 10.2 | 9.9-13.4 |
| aPTT | 28.6 | 24.0-39.2 |
| Bleeding time | 5.7 | 5.5+/-1 |
This case series expands the phenotype of OTUD6B-related disorders by suggesting a potential link between OTUD6B mutations and immune dysregulation. While the neurological features are consistent with previously reported cases, the novel observation of ITP raises the possibility that OTUD6B mutations may predispose individuals to hematologic autoimmune conditions. The involvement of OTUD6B in immune regulation, particularly in type I interferon responses, warrants further investigation to better understand its role in immune homeostasis. These findings highlight the importance of recognizing immune manifestations in OTUD6B-related disorders and underscore the need for larger studies and advanced molecular research to confirm and expand upon these results.
The absence of detailed immune assays represents a limitation of this report; nevertheless, the case highlights the importance of hematologic surveillance in patients with OTUD6B mutations. Multidisciplinary care, including hematology and immunology consultations in addition to neurology follow-up, should be prioritized to optimize long-term outcomes.
Biallelic variants in OTUD6B have been associated with an intellectual disability syndrome characterized by seizures and dysmorphic features. However, the potential link between OTUD6B mutations and immune system dysregulation remains unexplored. In this report, we present two sisters with a homozygous OTUD6B (c.173-2A>G) mutation, both exhibiting classical features of the disorder, with one uniquely manifesting ITP. This observation suggests a possible novel association between OTUD6B mutations and immune dysfunction.
OTUD6B mutations have been identified in diverse populations, including the Middle East, where consanguinity may contribute to a higher incidence of autosomal recessive disorders [2]. However, specific epidemiological data on the prevalence of OTUD6B-related disorders in this region remain scarce.
OTUD6B encodes a member of the ovarian tumor domain-containing subfamily of deubiquitinating enzymes (DUBs), which are critical regulators of the ubiquitin-proteasome system [2]. This system governs various cellular processes, including protein degradation, intracellular trafficking, and cell signaling. Disruptions in DUB function have been implicated in several human diseases, such as neurodegeneration, inflammation, and cancer [3]. While the neurological implications of OTUD6B mutations are well documented, their impact on the immune system has not been thoroughly investigated. The occurrence of ITP, a condition characterized by immune-mediated platelet destruction, in one of the sisters raises the possibility that OTUD6B mutations may contribute to immune system dysregulation. Zhao et al. performed a pan-cancer and immunogenomic analysis of OTUD6B, reporting its association with immune biomarkers such as IL-6 and TNF-α dysregulation, abnormal T-cell signaling, and altered tumor immune microenvironments [4]. Although their study was primarily oncologic, these findings support the hypothesis that OTUD6B dysfunction contributes to immune imbalance. In our patient, the occurrence of ITP aligns with the proposed immunologic role of OTUD6B and suggests that dysregulated cytokine production may predispose to autoantibody-mediated platelet destruction.
Emerging studies have linked OTUD6B expression to immune-related factors, including tumor mutational burden (TMB), microsatellite instability (MSI), the tumor microenvironment (TME), immune checkpoint genes, and immune cell infiltration. Experimental models in lung cancer cell lines further support its role in immune modulation Liu, et al. 2022. While these studies primarily focus on oncological settings, they highlight the broader immunological significance of OTUD6B and suggest its involvement in immune homeostasis.
Our findings expand the known phenotype of OTUD6B-related disorder by suggesting a potential role in immune regulation. While developmental delay, microcephaly, and seizures align with previously reported cases, the occurrence of Immune Thrombocytopenic Purpura (ITP) is a novel finding. OTUD6B has been implicated in immune pathways, including the regulation of type I interferon responses [5]. These findings raise the possibility that OTUD6B mutations may predispose affected individuals to hematologic autoimmune conditions, similar to mutations in OTUD1, which have been shown to enhance RIG-I-like receptors (RLR) signaling and disrupt IFN production. As said by Lu, et al. OTUD1 mutations attenuate its suppression of IRF3, leading to exacerbated autoimmune conditions [6]. Given the known role of Type I interferons (IFN-I) in promoting immune activation and autoantibody production, OTUD6B mutations could lead to aberrant IFN-I signaling, contributing to the generation of autoantibodies against platelets [7]. Animal model data, though limited, provide further context. Experimental knockout of Otud6b in mice has been linked to impaired lymphocyte maturation, altered interferon signaling, and increased susceptibility to immune dysregulation. Such findings imply that the gene plays a critical role in maintaining immune tolerance. Environmental factors such as viral infections may act as secondary triggers, further destabilizing immune homeostasis in genetically vulnerable individuals and leading to autoimmune cytopenias like ITP. This could promote platelet destruction and result in the development of ITP. Similarly, OTUD6B likely plays a pivotal role in maintaining immune homeostasis, with mutations impairing the regulation of immune activation and suppression. This suggests that OTUD6B dysfunction could increase susceptibility to hematologic autoimmune disorders such as ITP, highlighting the need for further research into its immune regulatory mechanisms [6,4].
Given that ITP involves aberrant immune-mediated platelet destruction, disruptions in OTUD6B-mediated pathways may contribute to immune abnormalities. Investigating the immunological functions of OTUD6B, particularly outside of oncological contexts, is essential to determine whether its deficiency predisposes individuals to immune-mediated diseases.
This report is among the first to propose a link between OTUD6B mutations and immune dysfunction, expanding the known phenotype associated with this genetic alteration. The comprehensive clinical descriptions of the case provide valuable insights into the variability and spectrum of manifestations in OTUD6B-related disorders. However, as these findings are based on a single family, additional studies are needed to confirm and generalize the association between OTUD6B mutations and immune dysregulation.
Overall, these findings suggest that OTUD6B-related disorders may have a broader phenotypic spectrum than previously recognized, encompassing not only neurological but also immunological manifestations.
Mechanistically, OTUD6B encodes a deubiquitinating enzyme within the ubiquitin-proteasome system. This pathway regulates protein stability in neurons and immune cells, directly influencing interferon signaling and immune activation. Loss-of-function mutations impair deubiquitination, causing aberrant cytokine secretion, impaired regulatory T-cell function, and a breakdown in self-tolerance. These alterations may explain the dual neurological and immune manifestations observed in our patient.
This study is limited by the small sample size, as it is based on a single family, and the findings may not be generalizable. The literature on OTUD6B’s role in immune regulation is still limited, and further research is needed to clarify its mechanisms. Additionally, due to limited advanced molecular laboratory resources in Palestine, we were unable to perform more detailed immune profiling or functional assays. These factors underscore the need for larger studies and better molecular tools to explore the immunological implications of OTUD6B mutations.
Figures have been refined to strengthen the clarity of clinical findings. The pedigree has been expanded to illustrate carrier status across family members, the purpura image has been adjusted for resolution and contrast, and the platelet trend graph has been enhanced with clearer axes and a stronger trend line.
This case underscores that OTUD6B deficiency may extend beyond its established neurodevelopmental phenotype to encompass immune dysregulation, with important implications for patient care. Recognition of this association is critical to guide surveillance strategies and ensure timely hematologic intervention.
Declarations
Ethics approval and consent to participate: This study did not require institutional ethical approval. Written informed consent was obtained from the patient’s legal guardian.
Consent for publication: Written informed consent for publication of clinical details and images was obtained from the patient’s legal guardian.
Availability of data and materials: (All data generated or analyzed during this study are included in this published article. Additional information is available from the corresponding author upon reasonable request.)
Acknowledgments: The authors acknowledge the assistance of the medical team at Jenin Governmental Hospital. We also appreciate the efforts and time of the patient’s family.
Authorship statement
Lilyan Jarrar, Ahmad Mashni, and Raya Fuqha contributed equally to this work and should be considered joint first authors.
Author contributions
All authors have made substantial contributions to the conception, design, data acquisition, analysis, and interpretation of data. They were involved in drafting the manuscript and critically revising it for intellectual content. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work.
- Bhattacharya S, Ghosh MK. Cell death and deubiquitinases: perspectives in cancer. Biomed Res Int. 2014;2014:435197. Available from: https://doi.org/10.1155/2014/435197
- Santiago-Sim T, Burrage LC, Ebstein F, Tokita MJ, Miller M, Bi W, et al. Biallelic variants in OTUD6B cause an intellectual disability syndrome associated with seizures and dysmorphic features. Am J Hum Genet. 2017;100(4):676–88. Available from: https://doi.org/10.1016/j.ajhg.2017.03.001
- Bello AI, Goswami R, Brown SL, Costanzo K, Shores T, Allan S, et al. Deubiquitinases in neurodegeneration. Cells. 2022;11(3):556. Available from: https://doi.org/10.3390/cells11030556
- Zhao G, Song D, Wu J, Yang S, Shi S, Cui X, et al. Identification of OTUD6B as a new biomarker for prognosis and immunotherapy by pan-cancer analysis. Front Immunol. 2022;13:944792. Available from: https://doi.org/10.3389/fimmu.2022.955091
- Wang J, Zheng H, Dong C, Xiong S. Human OTUD6B positively regulates type I IFN antiviral innate immune responses by deubiquitinating and stabilizing IRF3. mBio. 2023;14(5):e0218423. Available from: https://doi.org/10.1128/mbio.00332-23
- Lu D, Song J, Sun Y, Qi F, Liu L, Jin Y, et al. Mutations of deubiquitinase OTUD1 are associated with autoimmune disorders. J Autoimmun. 2018;94:156–65. Available from: https://doi.org/10.1016/j.jaut.2018.07.019
- Sehgal K, Guo X, Koduru S, Shah A, Lin A, Yan X, et al. Plasmacytoid dendritic cells, interferon signaling, and FcγR contribute to pathogenesis and therapeutic response in childhood immune thrombocytopenia. Sci Transl Med. 2013;5(193):193ra89. Available from: https://doi.org/10.1126/scitranslmed.3006277