More Information
Submitted: July 29, 2025 | Approved: August 05, 2025 | Published: August 06, 2025
How to cite this article: Narayana Swamy N. Oculomotor Palsy in a Subject with Thalamic Infarction: A Case Report. Arch Case Rep. 2025; 9(8): 258-260. Available from:
https://dx.doi.org/10.29328/journal.acr.1001155
DOI: 10.29328/journal.acr.1001155
Copyright license: © 2025 Narayana Swamy N. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Oculomotor palsy; Neuroophthalmology; Thalamic stroke
Oculomotor Palsy in a Subject with Thalamic Infarction: A Case Report
Nagalakshmi Narayana Swamy*
Ophthalmologist, Naruvi Hospitals, Vellore, Tamil Nadu, India
*Address for Correspondence: Nagalakshmi Narayana Swamy, Ophthalmologist, Naruvi Hospitals, Vellore, Tamil Nadu, India, Email: [email protected]
The thalamus is a highly complex nuclear structure comprising approximately 50–60 distinct nuclei, strategically positioned between the cerebral cortex and the midbrain. It plays a pivotal role in regulating sensory pathways, motor integration, consciousness, sleep, and cognitive functions. Through its vast interconnections, the thalamus serves as a key hub for neurocommunication, facilitating signal relay between cortical and subcortical areas.
This case report presents a rare instance of paramedian thalamic infarction selectively involving the dorsomedial nucleus of the thalamus, manifesting clinically as isolated oculomotor nerve palsy. Such presentations challenge conventional understanding of oculomotor dysfunction, which is typically attributed to midbrain lesions. The absence of brainstem involvement in this patient highlights the diagnostic importance of thalamic pathology in neuro-ophthalmologic syndromes. Our objective was to examine the visual and oculomotor manifestations associated with this atypical vascular event and assess clinical outcomes from an ophthalmologic standpoint. Key features analyzed include gaze abnormalities, ptosis, skew deviation, and pupillary asymmetry, all of which carry significant implications for quality of life and functional independence. The findings underscore the need for comprehensive neuro-ophthalmologic evaluation in cases of deep brain infarction, particularly those involving paramedian thalamic territory.
This report contributes to the growing recognition of thalamic strokes as important etiological factors in oculomotor dysfunction and provides insights into their clinical trajectory, prognostic markers, and rehabilitative strategies. Given their potential to mimic brainstem pathology, accurate early identification is essential for guiding neurorehabilitation and improving patient outcomes.
The thalamus is a major nuclear complex located in the diencephalon [1]. It serves as a relay centre for sensory, motor mechanisms, attention, and major neurocognitive processes. The thalamus is made up of a series of nuclei and is functionally divided into five components [2] and anatomically divided into three groups: relay nuclei, reticular nucleus, and intralaminar nucleus [3].
Stroke is an emerging cause of morbidity and mortality in developed countries and increasingly in low- and middle-income countries and contributing to the global health burden [4] thalamic infarctions account for 20% of all ischemic strokes and 14% of all lacunar infarctions [5,6].
Depending on the vascular regions and related nuclei, it causes a variety of neurologic symptoms. Thalamic infarction can cause a variety of visual and eye movement dysfunctions, either alone or in conjunction with infarction involving other regions, in addition to the traditional sensorimotor and amnestic syndromes [7-10].
There exists a lot of uncertainty around the underlying processes of oculomotor impairments, as well as possible future clinical courses and the most effective therapy strategy. Even in case series investigations of thalamic infarction with small samples, only a few have thoroughly addressed the neuro-ophthalmologic characteristics of the condition, despite the fact that numerous studies have been carried out to examine its clinical features [11,12].
The clinical diagnosis of thalamic infarction is challenging due to variations in anatomical location, volume, and lateralisation. This is a case report of paramedian thalamic artery infarction presenting with ophthalmoplegia.
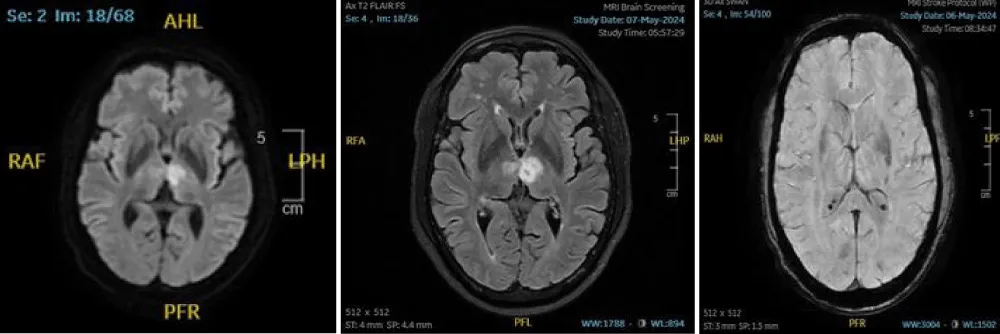
A 60-year-old male presented with a history of right-sided limb weakness accompanied by dysarthria. He had a known history of hypertension for six months. Magnetic resonance imaging (Figure 1) revealed an infarction in the left paramedian thalamus and the posterior limb of the internal capsule. MR angiography demonstrated an azygous anterior cerebral artery and a hypoplastic A1 segment.
Figure 1: An MRI image showing an acute left paramedian thalamic infarction.
Admission examination, the patient was drowsy but oriented. His blood pressure was elevated at 170/100 mmHg, and his Glasgow Coma Scale score was E4V4M5. General physical examination findings were within normal limits, and systemic evaluation was unremarkable. The electrocardiogram showed normal sinus rhythm.
Ocular Findings: Ocular examination revealed skew deviation with right-sided head tilt. Pupillary diameters measured 3 mm on the right and 4 mm on the left, with sluggish responses to light. The left eye showed restricted elevation, depression, and adduction, while the right eye was normal. Visual fields and posterior segment examination were within normal limits.
Neurological Findings: The patient exhibited central-type right facial nerve palsy without tongue deviation or limitation of tongue movement. No behavioral changes were noted. Limb power was rated at 2/5 on the right and 4/5 on the left. Babinski and Hoffmann reflexes were negative, and cerebellar function was intact.
A mild reduction in light touch sensation and proprioception was noted in the left upper and lower extremities. Brunnstrom recovery stages were graded as five for the left upper extremity, hand, and lower limb. However, manual muscle testing showed decreased strength in the left lower limb, with a grade of 3. Laboratory tests were within normal limits.
The patient was transferred to neurology care for further management.
The thalamus functions as one of the most critical relay centres in the brain. Its nuclei are categorized into four groups based on arterial supply: tuberothalamic artery, thalamogeniculate artery, posterior choroidal artery, and paramedian artery.
Paramedian arteries, a branch of the posterior cerebral artery, originate at the junction of the basilar bifurcation and the junction of the posterior communicating artery and supply the thalamus. Rarely, a single paramedian artery can supply both sides. If a single paramedian artery supply is present and stroke occurs, such patients present with coma, abnormal eye movements, and behavioural abnormalities.
Oculomotor nerve palsy is a common symptom of a midbrain infarction, but there have been reports of a paramedian thalamic infarction inducing third-nerve palsy without a definite lesion on the brainstem, as in the present case [13].
Elevation deficit is likely due to the involvement of the frontofugal dorsothalamic bundle transversing the mediodorsal nucleus and internal medullary lamina of the thalamus on its way to the superior colliculus in the midbrain [12,14]. Along with thalamic lesion, coexisting lesion involving the rostral interstitial nucleus of the medial longitudinal fasciculus in the upper midbrain may also account for the vertical gaze palsy. In the absence of a definite midbrain lesion in this case, medial gaze palsy could be due to the involvement of oculomotor nuclei or fascicle, associated with thalamic lesion extension.
Skew deviation can occur with lesions anywhere in the vestibulo-ocular pathway, which is also believed to traverse the thalamus. As with several previous studies, skew deviation was found in this study in more than half of the patients with paramedian thalamic infarction [15,16].
In addition to these features, ptosis [17-20], pseudo abducens palsy [21-23], Gaze-evoked nystagmus [24,25] have also been reported in previous studies.
A thalamic infarction not only causes motor impairments and sensory changes but also presents with oculomotor palsy, abnormal behavioural changes, and impaired executive function. Previous studies have reported sexually abnormal behaviour, sleep disturbance, amnesia, and the loss of self-activation in a paramedian thalamic infarction [11].
In the present case, ipsilateral oculomotor palsy appeared due to thalamic infarction, mimicking crossed hemiplegia observed in brainstem lesions. Although many oculomotor deficits resolve spontaneously within months, some may persist for years. Our patient received neurological care and showed gradual recovery over time—highlighting the importance of early assessment and targeted management in thalamic infarction.
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Child Nerv Syst. 2002;18:386–404. Available from: https://doi.org/10.1007/s00381-002-0604-1
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion: a functional MRI study. Brain. 1998;121:1281–1294. Available from: https://doi.org/10.1093/brain/121.7.1281
- Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264–2278. Available from: https://doi.org/10.1161/01.str.0000087786.38997.9e
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–255. Available from: https://doi.org/10.1016/s0140-6736(13)61953-4
- Yaghi S, Raz E, Yang D, Cutting S, Mac Grory B, Elkind MS, et al. Lacunar stroke: mechanisms and therapeutic implications. J Neurol Neurosurg Psychiatry. 2021;92:823–830. Available from: https://doi.org/10.1136/jnnp-2021-326308
- Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in understanding the pathophysiology of lacunar stroke. JAMA Neurol. 2018;75:1273. Available from: https://doi.org/10.1001/jamaneurol.2018.1073
- Caplan LR, DeWitt LD, Pessin MS, Gorelick PB, Adelman LS. Lateral thalamic infarcts. Arch Neurol. 1988;45:959–964. Available from: https://doi.org/10.1001/archneur.1988.00520330037008
- Saez de Ocariz MM, Nader JA, Santos JA, Bautista M. Thalamic vascular lesions: risk factors and clinical course for infarcts and haemorrhages. Stroke. 1996;27:1530–1536. Available from: https://doi.org/10.1161/01.str.27.9.1530
- Renard D, Castelnovo G, Campello C, Bouly S, Le Floch A, Thouvenot E, et al. Thalamic lesions: a radiological review. Behav Neurol. 2014;2014:154631. Available from: https://doi.org/10.1155/2014/154631
- Steinke W, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA, et al. Thalamic stroke: presentation and prognosis of infarcts and hemorrhages. Arch Neurol. 1992;49:703–710. Available from: https://doi.org/10.1001/archneur.1992.00530310045011
- Kim EJ, Kim MO, Kim CH, Joa KL, Jung HY. Abnormal ocular movement with executive dysfunction and personality change in a subject with thalamic infarction: a case report. Ann Rehabil Med. 2015;39:1033–1037. Available from: https://doi.org/10.5535/arm.2015.39.6.1033
- Lee HY, Kim MJ, Kim BR, Koh SE, Lee IS, Lee J. Acute pseudobulbar palsy after bilateral paramedian thalamic infarction: a case report. Ann Rehabil Med. 2016;40:751–756. Available from: https://doi.org/10.5535/arm.2016.40.4.751
- Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988;38:837–848. Available from: https://doi.org/10.1212/wnl.38.6.837
- Gentilini M, De Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: report of eight cases. J Neurol Neurosurg Psychiatry. 1987;50:900–909. Available from: https://doi.org/10.1136/jnnp.50.7.900
- Blitshteyn S, Hentschel K, Czervionke LF, Eidelman BH. Transient vertical diplopia and nystagmus associated with acute thalamic infarction. Clin Imaging. 2006;30:54–56. Available from: https://doi.org/10.1016/j.clinimag.2005.07.033
- Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex. Ann Neurol. 1994;36:337–347. Available from: https://doi.org/10.1002/ana.410360304
- Azabou E, Derex L, Honnorat J, Nighoghossian N, Trouillas P. Ipsilateral ptosis is the main feature of tuberothalamic artery infarction. Neurol Sci. 2009;30:69–70. Available from: https://doi.org/10.1007/s10072-008-0008-4
- Kim EJ, Lee DK, Kang DH, Ku BD, Kim JS, Na DL, et al. Ipsilateral ptosis associated with anterior thalamic infarction. Cerebrovasc Dis. 2005;20:410–411. Available from: https://doi.org/10.1159/000088664
- Averbuch-Heller L, Leigh RJ, Mermelstein V, Zagalsky L, Streifler JY. Ptosis in patients with hemispheric strokes. Neurology. 2002;58:620–624. Available from: https://doi.org/10.1212/wnl.58.4.620
- Topcular B, Yandim-Kuscu D, Colak M, Behrem N, Karagoz-Sakalli N, Gul G, et al. Unilateral ptosis associated with paramedian thalamic infarction. Ideggyogy Sz. 2011;64:275–276. Available from: https://pubmed.ncbi.nlm.nih.gov/21863696/
- Fisher CM. The pathologic and clinical aspects of thalamic hemorrhage. Trans Am Neurol Assoc. 1959;84:56–59. Available from: https://pubmed.ncbi.nlm.nih.gov/13823193/
- Leichnetz GR. The prefrontal cortico-oculomotor trajectories in the monkey. J Neurol Sci. 1981;49:387–396. Available from: https://doi.org/10.1016/0022-510x(81)90029-0
- Lindner K, Hitzenberger P, Drlicek M, Grisold W. Dissociated unilateral convergence paralysis in a patient with thalamotectal haemorrhage. J Neurol Neurosurg Psychiatry. 1992;55:731–733. Available from: https://doi.org/10.1136/jnnp.55.8.731
- Suzuki Y, Buttner-Ennever JA, Straumann D, Hepp K, Hess BJ, Henn V. Deficits in torsional and vertical rapid eye movements and shift of Listing's plane after uni- and bilateral lesions of the rostral interstitial nucleus of the medial longitudinal fasciculus. Exp Brain Res. 1995;106:215–232. Available from: https://doi.org/10.1007/bf00241117
- Helmchen C, Glasauer S, Bartl K, Buttner U. Contralesionally beating torsional nystagmus in a unilateral rostral midbrain lesion. Neurology. 1996;47:482–486. Available from: https://doi.org/10.1212/wnl.47.2.482