More Information
Submitted: July 09, 2025 | Approved: July 16, 2025 | Published: July 17, 2025
How to cite this article: Pulipati S, Puttagunta SB, Aswini S, Nandini Y, Lavanya K. Gut Microbiota in Health and Disease: A Path to Personalized Medicine. Arch Case Rep. 2025; 9(7): 231-244. Available from:
https://dx.doi.org/10.29328/journal.acr.1001152
DOI: 10.29328/journal.acr.1001152
Copyright license: © 2025 Pulipati S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Gut microbiota; Diversity; Irritable bowel syndrome; Personalized medicine
Gut Microbiota in Health and Disease: A Path to Personalized Medicine
Sowjanya Pulipati*, Srinivasa Babu Puttagunta, S Aswini, Y Nandini and K Lavanya
Department of Pharmaceutics, Vignan Pharmacy College (Autonomous), Vadlamudi, Guntur (Dt.), Andhra Pradesh, India
*Address for Correspondence: Sowjanya Pulipati, Department of Pharmaceutics, Vignan Pharmacy College (Autonomous), Vadlamudi, Guntur (Dt.), Andhra Pradesh, India, Email: [email protected]
This paper reviews groundbreaking research uncovering the precise dynamics of how nutrients in food affect the gut microbiota and how the gut microbiota in turn affects health in individualized ways. Throughout this paper, the nuances of these dynamics are explored, and the concept of precision nutrition and its practical implications are laid out. Microbes significantly impact how the body processes nutrients from food. It is clear that nutrients and chemicals in food directly influence the structure of gut bacterial communities, protecting against dysbiosis and benefiting health. However, it was not fully understood about the precise dynamics of how nutrients in food impact the gut microbiota and how these microbes in turn affect health, in individualized ways. Recently, in a series of ground-breaking studies, researchers filled in many details of this big picture, investigating detailed mechanisms of action and execution. This research offers the promise of personalized dietary guidance based on an individual’s unique gut microbiota. Microbes are deeply intertwined with health and well-being. The microbiome can be considered a vital organ because of its critical functions for the host. One crucial function is the role that commensal microbes play in the pharmacological bioactivation of inactive compounds, including drugs. This complicated series of actions further validates the need for a better understanding of the genetics in response to therapeutic agents.
Microbial interplays in the gastrointestinal (GI) tract shape the dynamics of gut microbiota in a complex and time-responsive manner. The succession of microbial populations in the gut is modulated by various factors, such as genetic and dietary aspects, that synergistically contribute to the balance of bacterial ecosystems. Recent developments in the field of modulating bacterial homeostasis open novel perspectives within gastroenterology. In this framework, precision nutrition is able to leverage gut microbiome uniqueness to counteract bacterial imbalances and, as a result, prevent or remedy GI disorders, as suggested by the recent literature [1]. In the era of precision medicine, the advent of -omic sciences, systems biology tools, and bioinformatics analytical pipelines has provided an unprecedented exploration capability of the molecular mechanisms underlying diseases. At the patient level, these new technologies make it conceivable to retrieve a comprehensive portrait of an individual (i.e., his genetic composition, metabolomics, proteomics, and gut microbiota), hence envisaging tailored therapeutic decisions on his features. In this view, it is expected that in the near future to assist a radical transformation of clinical practice will occur, moving towards personalized and evidence-based interventions [2,3].
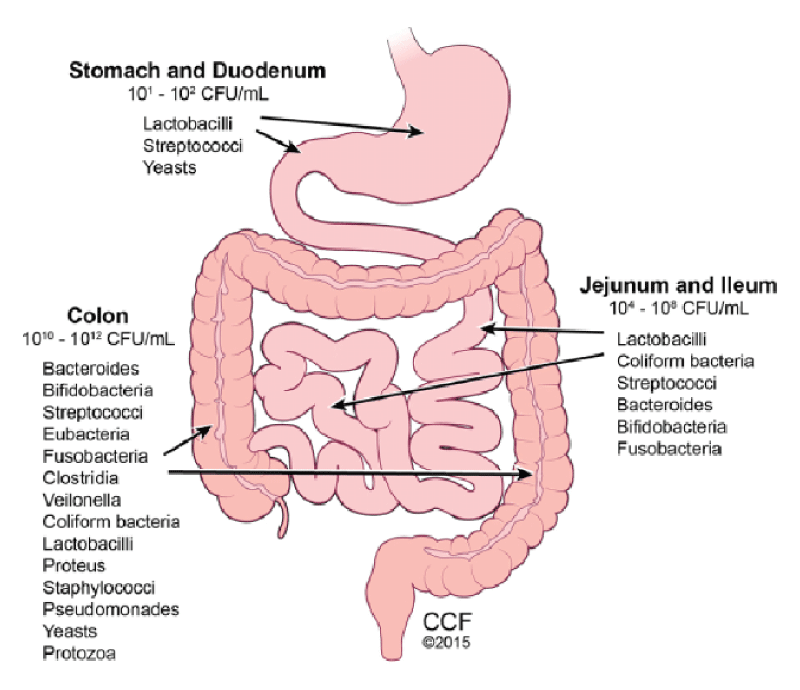
At present, only hospital research institutes, university hospitals, or health networks have the infrastructure required to systematize and analyze omics and clinical data. Meanwhile, some companies store information from millions of patients, with the hope of tailoring medical advice to protect and improve the health of each individual. Strikingly, all those-omics providers have chosen to neglect one of the most relevant drivers of individual differences in health, i.e., the gut’s ecosystem, known as the gut microbiota complex and dynamic assembly of microbes that co-evolved with Homo sapiens. Given the clear links between the gut microbiota and several diseases, and the malleability of the microbiota composition, the urgent necessity to include the intestinal microbial information in population studies is becoming. This is particularly important for therapeutic purposes because the gut microbiota-oriented personal interventions appear to be a potent and often underestimated tool in the hands of healthcare providers for the management of patient health [4,5] (Figure 1).
Figure 1: Human Gut Microbiota.
Overview of gut microbiota
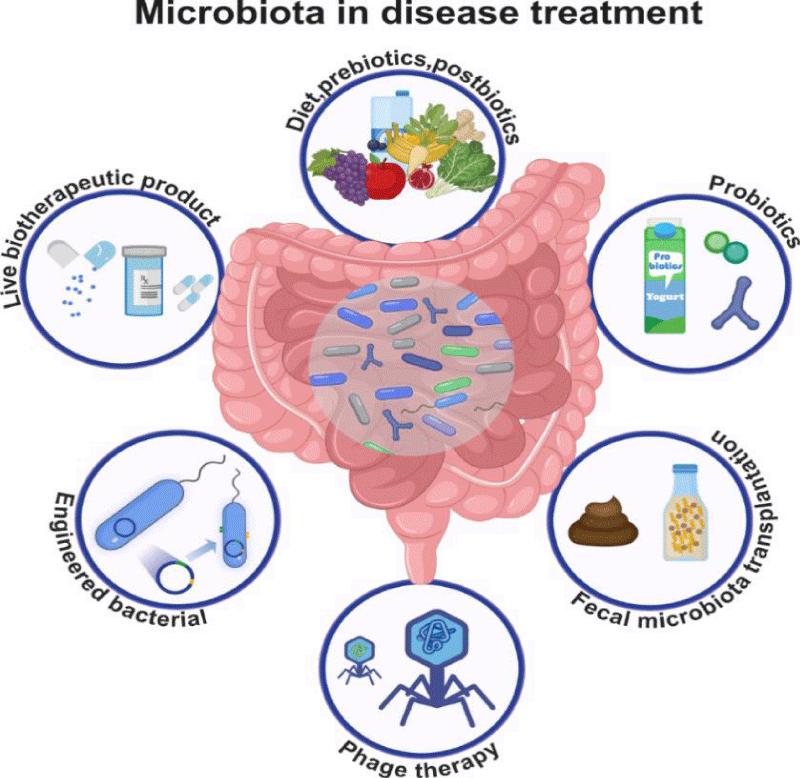
The Human gut is home to a complex and diverse microbial ecosystem, consisting of bacteria, viruses, fungi, and archaea, that substantially outnumbers our body cells. Fermentation of non-digestible food ingredients by gut microbes generates short-chain fatty acids (SCFAs), gases, and other metabolites, whose biological effects extend far beyond gut physiology regulation. It enters the host immune system, affecting the host physiology and homeostasis at local and distant tissues. For these reasons, the gut microbiota has been actively studied in recent years, and it is increasingly becoming a basis for many clinical interventions. Each individual is provided with a unique gut microbiota profile that plays many specific functions in the host. Host–microbe metabolic interactions are the best-characterized aspects of this complex crosstalk, but many other potential functions have been recently proposed, such as maintenance of the structural integrity of the gut mucosal barrier, immunomodulation, communication with the central nervous system, and protection against pathogens. Disease development is often associated with or triggered by an alteration in the human gut microbiota composition, a condition known as dysbiosis [6]. Intestinal disorders are the obvious consequences of gut microbiota imbalance, reflecting the primary location of gut microbes. However, dysbiosis is associated with numerous extra-intestinal diseases, including metabolic and neurological disorders. Despite the evidence of a tight relationship between gut symbiosis and health status, manipulation of the gut microbial community composition remains an intriguing terra incognita. Indeed, emerging evidence indicates that antibiotics, probiotics, and prebiotics, the current tools for the modulation of the gut microbiota, might not have a clear beneficial effect since their effects are dependent on the specific conditions and dose of the treatment. Unrealistic dietary interventions have also been proposed in the popular press, promoting the consumption of expensive food supplements supposedly promoting a healthy microbiota. Efforts are being made to develop novel therapies, such as fecal transplantations or bacteriophage therapies, but these approaches are sometimes risky. However, understanding whether dysbiosis is the cause or the consequence of disease might be a key step when addressing how to maintain or restore a healthy gut microbiota composition, leading to the possibility to interfere directly with the taxonomy of the gut microbiota by developing new therapeutic drugs [7-9] (Figure 2).
Figure 2: Strategies to modify gut microbiota for disease treatment.
Composition of gut microbiota
Each individual is provided with a unique gut microbiota profile, which is able to play many species-specific functions in host nutrient metabolism, maintenance of structural integrity of the gut mucosal barrier, immunomodulation, and protection against pathogens. Gut microbiota consists of various bacterial species, at least 100 trillion bacteria, with about 3 million genes, vastly outnumbering human genes. Microbiota predominantly features bacteria, with a ratio of 10 to 1 compared to human body cells. Microbial cells in the body exceed human cells by a factor of 10 to 100. This microflora accounts for 70% of the immune system and is crucial for biological processes linked to health. Research shows that intestinal microbiota plays a strategic role in human health and disease [10].
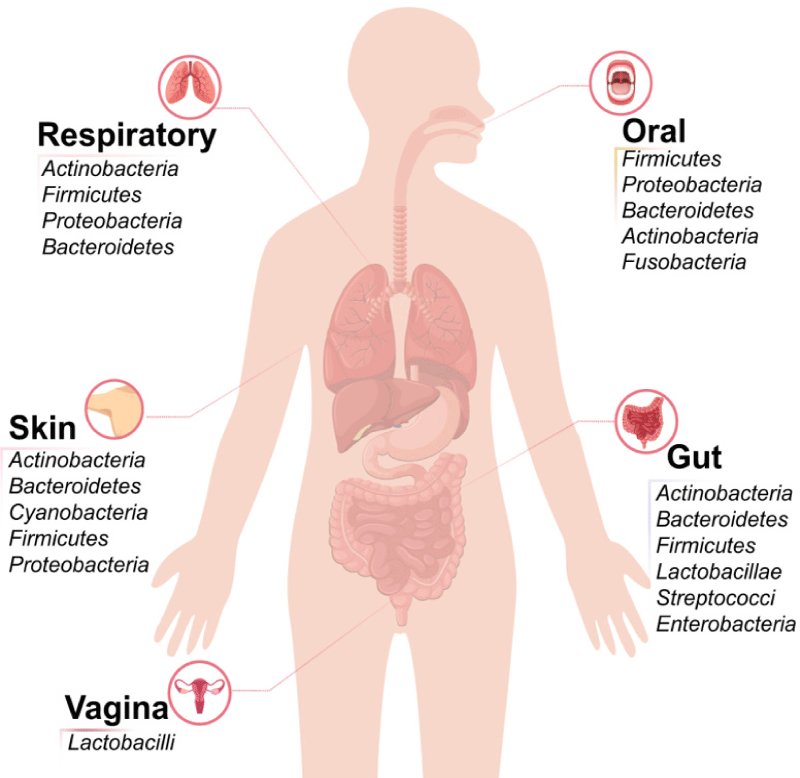
Each human’s gut microbiota is shaped early in life, with its composition varying due to infant transitions and external factors like delivery mode, feeding, probiotic use, infections, and antibiotics. Recent research indicates that individuals have a core native microbiota established in early life and maintained by the immune system and food. Despite the variability in composition among the population, gut microbiota serves similar basic functions across different individuals, stabilizing in adulthood. A study of 393 individuals highlighted interpersonal differences in gut metagenomic data linked to phenotypic variations, revealing three main enterotypes dominated by Bacteroides, Prevotella, and Ruminococcus species. Factors influencing the gut bacterial community include age, body mass index (BMI), exercise, lifestyle, gender, and geographical location, along with significant cultural and dietary habits. Consequently, an optimal gut microbiota composition is seen as a utopian goal since it varies for each individual. However, it is possible to define health and disease concerning gut microbiota. A healthy microbiota is characterized by a host-microorganism ecosystem without dysbiosis, meaning individuals may not exhibit specific diseases. Achieving a steady state in gut microbiota is advantageous for disease management [11-14] (Figure 3).
Figure 3: Human microbiota composition in different locations (oral cavity, respiratory tract, skin, gut, and vagina).
Functions of gut microbiota
Each individual has a unique gut microbiota profile, composed of various bacterial species categorized by taxonomic classifications that perform essential functions for host nutrient metabolism, structural integrity of the gut mucosal barrier, immune responses, immunomodulation, pathogen protection, trophic effects on the intestinal epithelium, and vitamin and amino acid biosynthesis. An individual’s gut microbiota composition is influenced by intrinsic and extrinsic factors such as genetics, diet, medications, chronic diseases, geographic and socio-economic health access, and urbanization levels. The gut microbiota, weighing around 1 - 2 kg, is shaped early in life, influenced by factors like birth mode and nutrition, and subsequently impacted by diseases and treatments. Over a lifetime, personal and healthy core native microbiota develop, mainly consisting of a small group of bacterial taxa, remaining relatively stable. Maintaining gut homeostasis relies on balanced interactions between gut microbiota, the immune system, and host mucosa. Enterotypes arise from different functional properties of gut microbiota rather than phylogenetic differences, displaying significant intra- and inter-individual diversity in these functional aspects. Ultimately, gut microbiota are crucial for metabolic, developmental, and immune processes as well as gut maturation and function, with contrasting effects between commensal and pathogenic bacteria [15,16].
Factors influencing gut microbiota
Each individual has a unique gut microbiota profile that serves specific functions related to metabolic, immune, and molecular aspects of health. This microbiota consists of various bacteria, classified by genus, family, order, and phylum. The beneficial gut microbiota includes native strains developed since birth, aiding in health maintenance. Gut microbiota begins to form in early life, influenced by breastfeeding, weaning, diet changes, and environmental factors, including parental influences and antibiotic exposure. These stable core microbiota remain largely consistent into adulthood but can be affected by significant disturbances. Each person’s gut hosts a slightly different array of microbial species, resulting in a vast catalog of bacterial strains. These communities cluster into stable patterns known as enterotypes. Disruption of beneficial strains can lead to dysbiosis, linked to various diseases. Additionally, gut microbiota are dynamic, varying between individuals and over time. An individual’s gut profile can inform disease susceptibility, supporting preventive and therapeutic applications through a multi-omics approach for integrated analysis of the host and microbiome [17].
The gut microbiota is a complex and influential ecosystem integral to living beings. It plays a vital role in immunity, digestion, metabolomic pathways, and the bioavailability of nutrients and drugs, and is linked to certain mental health disorders. A beneficial gut microbiota profile comprises indigenous and moderate strains that support the host’s ongoing gut microenvironment, contributing to immunomodulation and homeostasis. Various internal and external factors that shape beneficial gut microbiota are discussed, with reflections for future research. Animals raised in germ-free conditions show underdeveloped intestines, with shorter mucosal villi and absent lymphoid flora [18,19].
Gut microbiota and human health
Each individual has a unique microbiota profile composed of trillions of microorganisms that perform essential functions like nutrient metabolism, maintaining gut barrier integrity, immunomodulation, and protection against pathogens. A key element is the mucous layer covering the colonic epithelium, which serves as a passive barrier that enriches the ecosystem, allowing microorganisms access to the intestines. The gut microbiota includes over 1000 species, mainly bacteria from 4 phyla, influenced by diet. The GI environment can support stable bacterial populations with diverse biochemical properties. The host’s genetic background significantly impacts the microbiota profile, affecting the chances of specific microorganisms thriving within the ecosystem [20,21].
Recent mathematical modeling has explored the joint dependence of host and microbiota sides, focusing on two key aspects. It examines mechanisms that stabilize the dwelling biota and how ADM influences its dynamics. The study investigates how sociodemographic and lifestyle factors related to ADM affect gut microbiota diversification over time and the potential for reported preparations to cause dysbiosis. Ultimately, the findings enhance understanding of host-gut microbiota dynamics and highlight the risk of ecosystem collapse due to ADM. Optimization of host microorganism nutrients occurs when both components adopt a coherent evolutionary strategy. The stability of the microbial community plays a critical role in host metabolism and may contribute to health benefits [22,23].
Role in metabolism
The human gut hosts trillions of microbes crucial for health and nutrition. These microbes significantly influence host metabolism, providing functions lacking or underexpressed in the host’s genetic framework. Metagenomic and metatranscriptomic studies reveal the importance of key microbial species and the extensive metabolic pathways they maintain. These pathways are common in microbial communities and persist as a core component, even as their activity fluctuates with different dietary inputs [24].
Food serves as a key source of precursors for metabolite production, providing dietary molecules that microbes utilize in their metabolism. Diet influences the gut microbiota (GM) and the overall ecosystem due to micro- and macro-nutrient effects on microbial metabolic profiles. The gut is where the resident ecosystem metabolizes nutrients from ingested food within the gastrointestinal tract. In the holobiont system of the gut and its microbial community, diet is a crucial factor affecting the taxonomic and metabolic diversity of microbial consortia. Nutrients and dietary metabolites modulate phenotypes and enable the conversion of complex nutrients into metabolites not produced by the host. A significant research area in medical science focuses on the interactions between the GM and the host through various metabolic pathways. Microbiota members can produce bioactive molecules like lipopolysaccharides, short-chain fatty acids, secondary bile acids, and biogenic amines [25].
Immune system modulation
The gut microbiota has evolved to mediate commensal-biased immune homeostasis. Active systems in the host maintain this balance with the gut microbiota, significantly affecting the systemic immune system and the host’s overall health. Understanding this gut–immune balance is a promising area for personalized medicine [26].
Immune homeostasis is a dynamic state that enables a quick response to pathogens while preventing autoimmunity and maintaining tolerance for beneficial commensals. The immune response to gut antigens is influenced by mechanisms that uphold homeostasis. The microbiota affects the differentiation and activation of various immune cells in the gut, such as mononuclear phagocytes, innate lymphoid cells, and antigen-specific T cells. These cells are further modulated by the microbiota based on their interactions with different bacterial taxa. The immune system has receptors to detect bacteria, leading to vastly different responses to commensals and pathogens [27,28].
The microbiota’s ability to suppress gut inflammation highlights its role in preventing inflammatory responses. The gut is crucial for the immune system’s self-recognition, as it is rich in antigens. A healthy microbiota helps maintain immune balance, and dysbiosis correlates with immune system imbalances and various diseases. Understanding how microbiota dysbiosis influences disease progression is vital amid the growing knowledge of the human microbiota and the increasing rates of chronic gut inflammation diseases [29,30].
Impact on mental health
In recent years, growing attention has been given to the link between gut microbiota and mental health. Research suggests that the gut microbiota can impact the brain and mental well-being through various mechanisms, including signaling via the vagus nerve, modulation of neuro-immune pathways, alteration of tryptophan metabolism, regulation of neuroendocrine activity, and production of neuroactive substances. Moreover, gut microbes are capable of synthesizing and influencing neurotransmitters like serotonin, dopamine, and glutamate, all of which are essential for brain function and immune regulation.
In Western countries, a set of aesthetically appealing, clearly understood ‘icons of alert’ was designed to help health professionals recognise the potential for depressive disorder. A pocket full of memory aids to assist with recognizing the sick. The mnemonic icons were introduced to help conversation between the clinician and patient dyad regarding the potential use of specific blood measures as part of the treatment strategy for health issues, including depressive disorders. These measurements impact symptoms and treatment, including signs that health care providers watch to protect while sick. Frequently studied by non-clinical researchers, such studies may reveal biological correlates to depressive symptoms. Not dealing with the underlying causes of depression, perhaps, but these metrics could also illustrate comorbidities that may be treatable or the side effects of treatment, with the physical health implications of the management of depression (something appreciated by leading health organizations) taking a backseat [31,32].
Gut microbiota in disease
The gut microbiome significantly influences host physiology related to health and diseases like inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS), metabolic syndrome, and obesity, all associated with dysbiotic gut microbiota. However, the vast diversity of individual gut microbiota makes it tough to establish direct links between specific bacterial clusters and disease onset, complicating the development of universal treatments. Still, recognizing the microbiome’s health role opens doors for personalized nutrition and the use of probiotics, prebiotics, or synbiotics. Healthcare professionals can offer tailored nutritional advice to promote beneficial gut microbiota while avoiding disruptive foods, even post-antibiotic treatment. Although research on gut microbiota’s health and disease connections is growing, many studies are still emerging. Future advancements in translational research could lead to breakthroughs in personalized medicine, drug discovery, and health improvement. Precision nutrition studies could transform disease management strategies. For instance, research shows that customizing diets based on gut microbiota composition can be impactful in IBS treatment. While a low-FODMAP diet is effective for some, its success varies; 50% of patients show no improvement, but those with personalized diets based on microbiota had a 76% reduction in symptoms. This highlights the gut microbiota’s implications for future treatment strategies and its crucial association with gastrointestinal health, a key focus for ongoing research [1,34].
Dysbiosis and its consequences
After De Finetti conceptualized “human holobiont”, emerging from its widespread mutualistic, parasitic, and commensal interaction with microbiota, the scientific community has focused on the study of host-microbiota crosstalk. As a result, it has progressively become apparent that humans are not the primary agents in their physiological processes; such a realization has led scientists to investigate the basis linking disease pathogenesis to microbiota [33,34].
In this brilliant and vast framework, the current bacteriological knowledge of human digestive and vaginal microbiota, discretizing microorganisms into commensal, beneficial, and pathogen species according to their interactions with the human host, gives grounds for a bright yet still unfamiliar world of questions and expectations. Human disorders, typically morbid and chronic, are commonly associated with imbalances of microbial presence and abundance in the human body. On this basis, in recent years, the so-called “dysbiosis” term has emerged, indicating the variation in the composition of the microbial population, in which some taxa increase and others decrease. This state, especially present in the gut, has been associated with an increased representation of potential pathobionts (e.g., Escherichia spp., Klebsiella spp.), as well as a reduced representation of beneficial taxa (e.g., lactobacilli, bifidobacteria). Dysbiosis has also been associated with a reduction in the commonly accepted biodiversity of the microbiome [6]. Dysbiosis is conceived as a condition that is not only the presence/absence of key taxa, and keeping within a broad range of inter-individual variability. At the gap between a healthy/microflora-balanced condition and a diseased state, dysbiosis pertains to a condition associated with and/or confounding a disease or, at least, its increased risk. Consequently, the wide spectrum of existing microbial communities found in the human body, atherosclerosis, and gut diseases may not involve a single dysbiosis-fixed plot. First, the colonization of microbiota starts with the birth canal during the passage of the infant, and subsequently via the maternal areola [35,36].
Gut microbiota in inflammatory bowel disease
Dysbiosis of gut microbiota, i.e., imbalanced and altered gut bacteria as one of the triggering factors, has drawn more attention for its association with the host immune system in recent years. Since then, a number of studies have described a potential connection between dysbiosis and immunological changes leading to the onset of inflammatory bowel disease. Hence, understanding the gut bacteria and host immune interactions will provide further insight into the treatment of inflammatory bowel disease. In this context, this study will provide a comprehensive overview of the interactions and dysbiosis between gut bacteria and the host immune system in inflammatory bowel disease and how the imbalance in gut bacteria might cause immune activation, thereby perturbing mucosal immunity in the intestine. It will also review current and future therapeutic strategies for inflammatory bowel disease with the goal of modifying gut microbiota by elucidating the interrelation and immune regulation network between host and gut bacteria [37,38].
Dysbiosis refers to an imbalance in the abundance of microbial species. Typical dysbiosis is accompanied by impaired gut barrier function and associated inflammatory/acquired immunity that is tailored for stimulation aimed at it. Major traits of dysbiosis in inflammatory bowel disease include loss of beneficial microbes, expansion of pathobionts, and loss of microbial diversity. Dysbiosis of gut bacteria, especially in the mucus layer of the intestine, is associated with the onset of inflammatory bowel disease. Among several observations, reduced diversity of gut microbiota is associated with the initiation of inflammatory bowel disease. Additionally, during and after inflammatory bowel disease manifestation, a series of studies have described alterations in the gut microbiota, although contradictory results remain. Meta-analysis approaches demonstrated a higher abundance of Enterobacteriaceae concurrent with a lower abundance of bacteroidetes, which is one of the most enriched phyla in Verrucomicrobia, Proteobacteria, and Actinobacteria, in patients with inflammatory bowel disease [38-40].
Role in obesity and metabolic disorders
The human organism is known to be composed of many human cells that constitute the tissues and organs. However, contrary to earlier beliefs, studies suggest that only a negligible 43% of the cells are of human origin, making us the host to a vast amount of microbes inhabiting the mucosal surfaces especially, from the oral cavity to the stomach, through the small and large intestines up to the rectal area [40]. These microbial forms include bacteria, viruses, protozoa, and fungi, and are referred to as the microbiota. Microbiota are vital to certain bodily functions, even though their structures remain unclear in healthy individuals, and research on this subject has only seen exponential growth.
The gut microbiota plays a significant role in both health and disease and is associated with various pathologies. In the past decade, technological advances in microbiota research have allowed analysis of the overall and associated functions of the gut microbiota, with a connection to the host’s health status and disease. The interactions between the gut microbiota and the host are dynamic and may become imbalanced due to various reasons. The changes can induce various disorders, and microbiota alteration or dysbiosis causes many diseases, including obesity and metabolic disorders. The microbiota is understood to have an impact on the metabolism of multiple nutrient classes. It has a significant effect on the concentration of metabolites such as short-chain fatty acids, bile acids, and branched-chain amino acids that are essential for host physiology. This adjustment with metabolites allows the various microbiota to influence host systems, affecting the metabolism of the host and increasing energy intake from the diet, thus contributing to the growth of the host. There is potential for the development of diagnostic approaches, personalized curing, and theranostic therapy in this context. Personalized curing of microbiota can be used to improve immune response, regulate allergic reactions, and fight against pathogens. This would enable an improved understanding of the individual’s health and disease choices while raising the quality of life of the people [41-43].
Association with autoimmune diseases
Previously, there was a reductionist conceptualization of autoimmunity as being driven by genetic predisposition, and T and B cells identifying “self” proteins. As incidents of autoimmunity have increased, this explanation has become inadequate. Within the realm of chronic diseases but outside of autoimmunity, more have arisen than can be explained by genetics, so doctors turned to epigenetics. Yet, as autoimmunity skyrockets, unaffected by these changes, and with as many environmental factors having proven protective as detrimental, this explanation, too, is insufficient. Based on epidemiological studies and burgeoning mechanistic research, an expansion of the view of autoimmunity is suggested, in which the firsthand breach in tolerance was dysbiosis of the gut microbiome. The impairments in barrier function and immune response induced by this dysbiosis are not simply a result of immune activation driving a feed-forward loop further depleting tolerance. Rather, in coherence with the initial autoimmunity arising, the host and microorganisms engage in a subtler “dance,” each shaping the other. Since the implications would drastically alter research into autoimmunity and the development of therapeutics, a fresh analytic perspective on the diseases and their potential causes as present is proposed. Rather than strictly “the immune system fails,” it takes the stance that it oscillates, with periods of hyperactivity leading to disease, and periods of hypoactivity with disease remission [44]. The latter state has similarities to a pro-inflammation state in short periods, relieving symptoms. Instead of the commonplace notion of the ill-timed immune attack on “self” proteins, it is proposed that dysbiosis of the microbiota is the root of all autoimmune diseases. This dysbiosis strongly influences barrier function and immune response, affecting immune components of both gut and glands [45-47].
A landmark study reflects the complexity of microbiota-autoimmunity relationships. NOD mice exposed to LPS, while developing leaky gut and altered microbiota, lead to protection from T1D with increased Tregs in the pancreatic lymph node (PLN). But NOD mice treated with an oral antibiotic cocktail raised neonatal animals in germ-free conditions, resulting in diminished autoimmune responses in the pancreas. However, when the same treatment was conducted in mature mice, it induced gut inflammation and autoimmune reactions in the pancreas. As a definite demonstration, gut environment plays a crucial role in the development of autoimmune insults in the pancreas. Additionally, the gut environment differently affects the initiation of autoimmune attacks depending on when in life it begins, for antibiotic-induced microbiome alterations take up to 9 weeks to reach the gut and 16 weeks to stabilize. A common misconception purports that it is possible to design a diet-based intervention that terminates specific microbial populations, as commercial probiotics and postbiotics cannot parallel the complexity and resilience of the zoonotic milieu, and dietary interventions of this nature do not have substantial or easily replicable effects. Far greater knowledge about how particular diets influence the microbiome is a prerequisite for realizing dietary treatments of this kind [48-50].
Microbiota and cancer
Multiple studies confirm gut microbiota association with neoplastic diseases. Breast cancer abrogates Lactobacillus and biotransforms estrogens, contributing to mammary tumor growth. The “driver-passenger” model coherently relates the CRC sequence to specific oncogenes and microbiota shifts. The evidence in CRC shows that drivers within oncogenes KRAS, GNAS, and BRAF increase stemness and Wnt pathway activation, selectively triggering expansion of intratumoral Fusobacterium. Passenger oncomutations, also increasing stemness, affect other genes and exert opposing selection pressures, leading to different intratumoral bacterial populations. Generally, intoxicated by FadA, these coexist with F. nucleatum throughout carcinogenesis, bridging driver passengers with later changes. Once established, bacterial subpopulations maintain their tumorigenic potential. Sporadic events and a heterogeneous luminal environment beget multiple primary tumors, including frequently observed KRAS-triggered non-invasive neoplasias that display the same characteristics of the CRC precursor. Subsequently, convergent acquisition of Wnt pathway-unleashing alterations is more frequent in lesions with a permissive colonic subregion colonized by oncogenic E. coli. Importantly, already prevalent E. coli in neighboring dysplastic foci facilitate the rapid emergence of such alterations. Collectively, these analyses describe a poly-step progression fully accommodating experimental and clinico-pathological observations on CRCs from humans and animal models alike. These results implicate bacteria in the early steps of neoplasia and establish the gut as an example of how outside factors can influence the sequence of events driving malignancy [51-53].
Personalized medicine approaches
Microbes inhabit various parts of the human body, including the gastrointestinal (GI) tract; approximately 95% of the microbes live in the GI tract. The gut microbiota increases the bioavailability of food ingredients and is involved in the metabolic transformation of food ingredients. Maintenance of gut microbiota homeostasis is closely related to human health, and an imbalance is observed in several diseases such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), obesity, and type 2 diabetes (T2D). In general, intrinsic factors such as gender, ethnicity, and age affect the composition of the gut microbiome. Among Korean adults, ethnicity has been reported as the most important factor identified in analyses of microbial community variation. Characterization of the gut microbiota has been performed in different countries, and the results have shown that Japanese people harbor an increased level of Bacteroidetes compared with people of other ethnicities [54]. With advances in sequencing techniques, studies analyzing human gut microbiota have been conducted before and after migration, particularly in individuals moving from Africa and Asia to Europe and the United States, to identify the preconditions that affect the gut microbiota. The gut microbiota composition changes after exposure to a Western lifestyle, with an increase in Bacteroides and a decrease in Prevotella. Different dietary habits according to geographic region and country are closely associated with differences in the microbial composition of the gut. Diet is a major environmental factor that shapes the gut microbial communities and is related to a multitude of microbiome-related conditions and illnesses. High microbial diversity and functional redundancy are considered to be healthy microbiomes. In addition, fiber degrades faster inside the intestines of Africans than in Europeans [55]. Therefore, the function of intestinal microbes in Africans is better adapted to degrade fiber. Although many bacteria are better able to digest fiber, butyrate produced in response is a major source of nutrition for the intestines. African Americans had a three times higher incidence of colon cancer than rural South Africans, but the disease was rare because the gut microbiota produced a protective metabolite. Advertising treatments to other countries increases the rate of the other country’s disease. Antibiotics are a common cause of dysbiosis in Western populations. In particular, the use of ceftriaxone has been associated with the suppression of beneficial gut bacteria even years after medication.
Microbiome profiling
In recent years, there has been increased interest in research on the role the human microbiome plays in health and human diseases, including metabolic, allergic, inflammatory, infectious, neurodegenerative, and autoimmune diseases, as well as cancer development. Advances in high-throughput omics technologies have paved the way for the rational utilization of microbiome profiling in studying disease etiology and progression. They opened the door to a comprehensive assessment of microbial composition and function, and facilitated insight into the biological role of the human microbiota in health maintenance vs. disease development [56]. As a result, the elucidation of a network of interconnected pathways linking the gut, brain, lungs, skin, mouth, and liver, the immune system, and metabolism in health and disease has broadened the spectrum of research that embraces a multidisciplinary approach. About an arm’s length of intestines lie inside the human body, with a single layer of cells separating their lumen from the remaining organs. Along the course, countless microbes, bacteria, viruses, parasites, fungi, and beyond constantly exchange signals with each other and their host, modulating a plethora of bodily functions such as digestion, absorption, and immunity. These interactions can be both beneficial, supporting digestion and the resistance to pathogens, or detrimental and underlie numerous conditions that vary, among others, in severity, duration, and mechanisms of the onset. Consequently, commensals and other gut inhabitants can drive metabolic, allergic, inflammatory, and infectious diseases as well as cancer development.
Targeted interventions
Dietary impact on gastrointestinal health is reviewed with a focus on precision nutrition. Recent studies of gut microbial interactions are highlighted. Personalized nutrition is emerging as a powerful approach to modify the course of gastrointestinal disorders [1]. Sickcare is currently more reactive than proactive. By the time symptoms manifest and a diagnosis is given, the disorder may have progressed to a severe stage. Rising healthcare costs combined with an aging population make this “sickcare” model unsustainable. Based on indicators like genetic predispositions or microbial composition, healthcare may be anticipated and livelihoods amended [54]. Of note, nutritional treatment accounts for a large part of the overall costs, but the effectiveness of the proposed diet is not always significant. To improve results, microbiota-adapted dietary strategies can be crafted. Such strategies may recommend foods that particular bacteria like, or those that may disrupt the balance. Gastrointestinal disorders will be at the heart of this review, but most of the spotlight will be on irritable bowel syndrome (IBS), as precision nutrition was predominantly explained within its limelight. It has been demonstrated that tailored dietary strategies matching the patient’s gut microbial profile considerably increase the effectiveness of dietary interventions, even in the difficult-to-treat case of the low-FODMAP diet. Experimental and translational approaches have made it possible to better understand disorders and, in certain cases, to create individual-specific therapies. Being at the crossroads of immunology, dietetics, and surgery, modern cancer is on the cusp of a revolution. Unprecedented advances in cellular and molecular biology even make it possible to envision the development of “strictly” personal drugs based on the pathology of the tumor or even the composition of the gut microbiota. Broad and particular flexibility presupposes replacing the approach to disease by a principle of health management, taking into account the genetic, physiological, and psychological characteristics of each individual. With this in mind, it is possible to achieve a realignment with physical, mental, and social well-being by popularizing the switch to benefit from adapted dietary plans from childhood.
Probiotics and prebiotics
There’s evidence that gut bacteria can influence weight, among other beneficial effects. Beneficial bacteria are also being used to make so-called probiotics, which have been shown to help general health or treat bowel problems. Prebiotics are used as food for beneficial bacteria, which can also be used to prevent bowel problems. Probiotics are live microbial foods or supplements, ingredients that promote health in the host, which means they form good bacteria in the intestines. Experiments show that a group of a hundred obese people were given either a placebo or probiotics. These people then continued to eat similar types of food but found that over 3 months, the group taking probiotics gained an average of 50% less weight compared to those given the placebo pill. Probiotic supplements or foods that contain them have been found to improve or help maintain general health, especially gut health. They have been associated with reductions in the number of ill days, as well as reduced numbers of sick people and lower numbers of doctor visits, because they are producing a better immune response. There are a number of studies that have shown the clinical efficacy of probiotics for various gut-based disorders like inflammatory bowel disease (IBD), diarrhea, and for digestion. Because of these observed effects, more studies have been carried out on probiotics, and many pharmaceutical or dairy-based products are on the market for the treatment of such illnesses or to maintain general health. Prebiotics are nondigestible oligosaccharides that provide food for beneficial bacteria. They are naturally found in garlic, agave, jicama, and some other plants and herbs. They have been used for centuries in traditional medicine. A review found that supplementation with prebiotics reduced the number of people who have episodes of diarrhoea. Other studies have shown that prebiotics can help in maintaining gut health and alleviate a number of gut predisposing disorders. Since they are natural products and act similarly to dietary fiber, they have become quite popular among medical doctors and people with gut-related illnesses, used as a dietary supplement [57].
Fecal microbiota transplantation
Due to its high safety profile, human stool for use in fecal microbiota transplantation (FMT) has been classified as a “biological product,” and research on FMT has been classified as a “biological treatment” that must be approved. FMT has been classified as “new” in comparison with the other classifications of treatments. When a patient receives an FMT, the donor microbiota profile becomes more similar to the recipient’s microbiota profile over time, and the recipient microbiota profile becomes more dissimilar to its pre-FMT state. This occurs as microbiome communities undergo rapid rearrangement after a perturbation, and the healthy microbiota from the donor takes over the perturbed states of the recipient [58,59].
As a new therapy for various diseases, FMT is still, for the most part, in an exploratory or developmentally focused pre-investigation stage. Therapeutic effectiveness has been associated with FMT for diseases that arise due to an unhealthy microbiota and are associated with an improvement following transplantation treatment. Although various strategies have been used to modulate the composition of the altered dysbiotic microbiota, success rates are varied, and for some diseases, therapeutic efficacy is still lacking. FMT as a biological system determines that entire microbial communities will be transferred from healthy donors to individuals suffering from diseases associated with an altered microbiota in efforts to recover from dysbiosis and ameliorate conditions associated with the disease. The transplantation of an entire ecosystem of gut microbiota has the advantage of delivering the human body an entire gut micro-ecology that properly functions as an organ, as opposed to the one-microbe-one-effect strategy followed in probiotics and the resulting mixture of communities that individualizes and allows for the proliferation of such piles of colonies. FMT is highly safe, with side effects predominantly limited to immediate issues, has no risk of rejection, and an immune response is not typically elicited between the recipient and donor. Before transplantation within the recipient, fecal stool from chosen donors needs to be manipulated on behalf of processing, preparing the product, and ensuring therapeutic quality [60,61].
Challenges in microbiota research
Microbiota research in the past two decades has included the data-driven coining of the term gut microbiota dysbiosis, which implies there is a microbial cause and that this is a sickening of the normal state. Given the phenomenally high inter-individual variability of the gut microbiota, the very notion of a normal microbiome state defies a profound understanding. Attempts to draw a link to a phylogenetic state have analysed a biologically defined healthy reference state, dating from a time when the microbiome was only seen as the sum of the microbes present in the human ecosystem, with no functional perspective. The microbial aspect of human biology has changed profoundly since the onset of microbiome research, but the formulations of health have not yet experienced a commensurate modification, with “healthy” operational definitions remaining at best vague, limited, situation-dependent, and generally reaffirming the pre-microbiome research bio-medical wisdom [62].
There are still major gaps in our understanding of microbial ecology in the human gut, which is a prerequisite for advancing microbiome research. Longitudinal microbiome studies are urgently needed that include a comprehensive mapping of the spatial differences as a baseline. Finally, one should realize that clinical intervention trials are the critical test for the practical value of microbiome research. There is much more at stake in simple applications than boosting health to investigate whether a particular diet can alter the gut microbiota so that the utilization of the drug Xmab in cancer treatment becomes more efficacious. Some of the seemingly very simple microbial questions around food are far from trivial, and one may have to accept that single drugs based on microbiome knowledge will always remain an exceptional case amidst a multitude of comparatively modest interventions. “Precision nutrition” may therefore find a market niche as a high-profile fad without fulfilling the high expectations raised by early exaggerated promises [63,64].
Variability among individuals
The healthy human gut microbiota is the composition and functions of the gut microbiota in the absence of disease. The gut microbiota consists of several species of microorganisms, including bacteria, yeast, and viruses. It is characterized by a large proportion of Firmicutes (64%) and Bacteroidetes (23%), followed by broad groups of Proteobacteria (8%) and Actinobacteria (3%), while less abundant phyla include Fusobacteria and Verrucomicrobia [10]. This composition is not homogeneous throughout the length of the gastrointestinal (GI) tract. Consequently, the general meaningful information provided will be general and refer to the intestines.
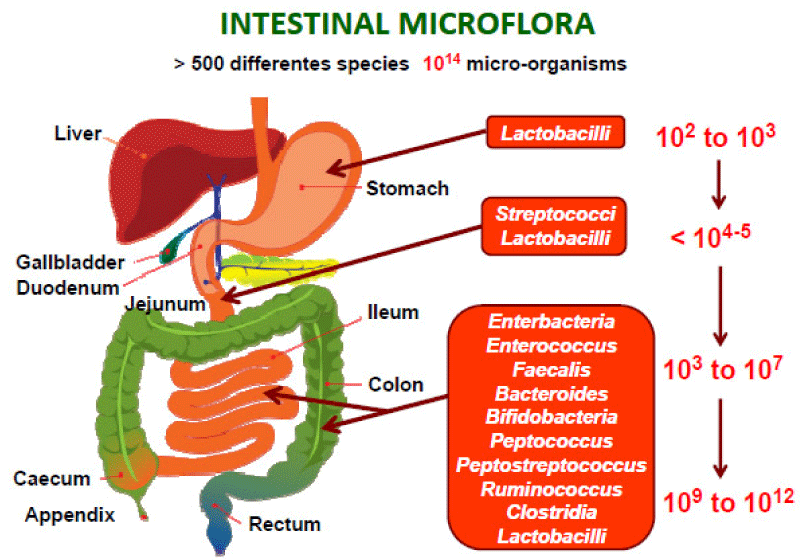
A complex community of microorganisms that inhabit the GI tract of humans. Studies have shown that such microbial communities are stable among individuals but can vary between different individuals. The variations can include, but are not limited to, “differences in the relative abundance of species or genes, the presence or absence of particular organisms or genes, or differences in the arrangement of genes in the genomes of one or more community members”. A large component of inter-individual variation is controlled by the person’s genetic makeup. Other factors that have been associated with a significant portion of variation in composition of the gut microbiota among individuals include age, diet, and environmental exposure [65] (Figure 4).
Figure 4: Spatial and longitudinal variations in microbial numbers and composition across the length of the gastrointestinal tract. The human microbiota contains as many as 1014 bacterial cells, a number that is 10 times greater than the number of human cells present in the body. The number of bacterial cells present in the mammalian gut shows a continuum that goes from 102 to 103 bacteria per gram of contents in the stomach and duodenum, progressing to 104 to 107 bacteria per gram in the jejunum and ileum, and culminating in 109 to 1012 cells per gram in the colon.
Ethical considerations
Besides the global human microbiome network, there are distinct projects for indigenous populations, as they show traditionally acquired microbiomes that contrast with western urbanized populations. Ethical considerations for microbiome research have been broader on the human microbiome, most importantly with a focus on biobanking and knowledge management. A panel representing diverse scientific and stakeholder communities has addressed microbiome research ethics. A set of guiding principles has been compiled, and they are joined with guidelines for compliance and enforcement, which can provide a more or less strict route for legitimate access to materials and on data handling and use, respectively. However, regardless of being a living organism or source of genetic information, ethics of microbiome research, and knowledge-derived use broadly concern other types of research and embraced policy areas related to medicine, intellectual property, legal, governance, and environmental protection. Basically, ethical aspects related to microbiome research and microbiome knowledge-derived use have thus far been most extensively considered in the context of human microbiome research and medicine [66]. A panel assembled to address a broader spectrum of ethical issues is encouraged by the global human microbiome research community. Microbiome knowledge is accumulating at a very fast pace across environmental ecosystems, animals, and humans, in healthy and disease-related conditions. The knowledge base is showing that microbiomes crucially influence health, nutrition, immune system function, growth, mental development, cognition, behavior, protection from pathogens, harm from infections, and toxic substances, and most of these effects are due to their metabolites. Moreover, the microbiome is demonstrated to have multifaceted interactions with host nutrition and the immune system [67].
Regulatory issues
Against the backdrop of unprecedented technological progress, the future of personalized medicine is fostered by the accrual of big data. Such an appraisal is supported by the microbiome and its modulatory effects on the pathophysiology of the human body. In addition, the uniqueness of the microbiome could constitute the wellspring for its incorporation into the matrix of emerging personalized dietary and pharmacological interventions. Nevertheless, the development of each novel form of intervention shall be preceded by profound familiarization with the factors modulating the homeostasis of the microbiome. A particular focus is placed on the gut microbiota, which is of utmost importance, as its equilibrium unbalances the preservation of one’s health. The present review delineates the constructs of the principles and nuances amplifying the dynamics of bacterial interactions within the gut. The mutualism of the microbiome upholds the proper functioning of the human body. Despite its complexity, several regulatory mechanisms are present, ensuring the purity of the bloodstream. One is the intestinal immune system, preventing the occurrence of constantly surfacing pathogenic bacteria, which might infect the organism. Nourishing fibers could conversely modulate the microbiome, fostering alterations that culminate in the boosting of the human body’s fight against pathogens. Subsequently, their growth results in an escalation in the concentration of diet and bile acids, ultimately leading to the proliferation of gut epithelial cells. However, technological innovations have contributed to the elucidation of finer/latent nuances, endowing one with a better understanding of the microbial interactions within the gut. In vitro cultivation could be replaced with the next-generation sequencing of nucleic acids, allowing the reconstruction of the already culminated interactions. From a broader scope, the clinical paradigm embraces the onset of the ‘omics era, connoting the developments in genomics, proteomics, etc., and their successive utilization in the apprehension of the particular course of disease onset [42,43].
Future directions
The burgeoning field of research devoted to gut microbiota and its interaction with the host has seen a steep rise in the past decade, offering new horizons in our understanding of human health and disease from the standpoint of microbial ecology. Although many studies have capitalized on the association between human gut microbiota and disease, research linking gut microbiome to the determination of treatment efficacy and dietary responses at the level of the individual is still scarce. That is the view taken in this review, attempting to delineate the way microbiota may fill a diagnostic and predictive niche in the approach to personalized medicine. Next, it delves into methodological issues and attempts to systematize the steps essential for the future translational research into practice. The prevention of multifactorial diseases such as colorectal cancer and cerebro-cardiovascular diseases through lifestyle changes can now be further explored using large-scale cell-type-specific computational model analysis. This will facilitate experimental planning for the evaluation of new prophylactic therapies, offering an extraordinary potential impact both for research project development and for public health.
Technological innovations in the last decades have paved the way for large-scale hunting of the uncharted diversities of the human microbiome and have contributed substantially to a deeper understanding of its role in health and disease. Insights from a collection of studies have shown that gut microbiota encodes a vast array of functional pathways, which also involve various metabolic processes. Disturbance of the intricate ecosystems that comprise the human microbiota, collectively termed dysbiosis, has been implicated in the etiology of many disorders, for example, gut diseases, that as mostly inflammatory bowel diseases or IBD and irritable bowel syndrome. Dysbiosis is a potential indicator of pathology, but it is possibly also a determinant of treatment discomfort because it is known to significantly affect drug metabolism and to influence the bioavailability of environmental chemicals. On the other hand, the cellular communication network is a complex 3D system consisting of a variety of biological entities and their interactions, making it difficult to understand. Ongoing efforts to consider this complexity are directed toward the development of computational models suitable to describe the systems biology of cell-to-cell communication [68,69].
Advancements in technology
The human gut is inhabited by a complex community of microorganisms that show both mutualistic and commensal relationships with the host. Gut microbes can provide a number of health benefits to their hosts, including the production of vitamins, hormones, and secondary metabolites, the digestion of otherwise indigestible polysaccharides, and the modulation of the immune system. Because of the myriad mutualistic roles played by commensal microorganisms in the gut, they have often been regarded as a hidden organ of the human body. Advances in DNA sequencing technologies have prompted gut microbiota to become the subject of intense research. New sequencing platforms and downstream bioinformatics pipelines allowed researchers to carry out comprehensive metagenome-sequencing analyses, apt at unraveling the metagenomic potential of the complex ecosystem of the gut microbiota [70,71].
Alterations in gut microbiota composition, or dysbiosis, have been associated with a number of health conditions. Because of the advent of targeted sequencing approaches, of growth media capable of isolating new and thus far uncultured microbes, as well as of metagenome-assembled genomes (MAGs), researchers discovered a wider array of commensal microorganisms residing in the human gut. Gnotobiotic animal models and metagenomic insert clone libraries shed some light on the physiological implications of the abundantly present and accurately cultured microbes. Still, the vast majority of microorganisms are not viable outside of the gut environment, and many of these microbes are still poorly understood in terms of metabolic and ecological relationships [72]. This knowledge gap, in tandem with the impossibility to study these microbes in a laboratory setting, prompted the development of novel bioinformatics approaches to bridge the gap between gut metagenomic sequences and the metabolic/functional role of the microbes they stem from.
Potential for disease prevention
In recent years, the emergence of personalized nutrition as a reaction to the paradigm “one size doesn’t fit all,” in combination with the growing recognition of the importance of the individual gut microbiome profile, gave rise to a novel area of study – precision nutrition. Tailoring nutrition to the individual’s microbiome might help to understand the inconsistencies between results obtained by studies and improve the prediction of response to diet [25]. Indigents in food, diet manipulation, and probiotic supplementation influence the state of the gut microbiota. Therefore, modulating diet to achieve a desired healthy gut microbiome state offers an opportunity to prevent, promote, or inhibit the development of various diseases [1].
The increasing number of investigations and the enhancement in techniques afford the chance of an extensive understanding of the gut microbiome and its correlation with health and disease. Armed with this information, healthcare professionals are able to offer personalized nutrition readings on the food requirements and prevent the outbreak of particular diseases. Changes in microbial diversity have been associated with diseases like obesity, metabolic syndrome, cancer, and autoimmune diseases. Therefore, by means of an early intervention plan, the outbreak of one or more diseases can be reasonably prevented [73,74].
A particular individual’s diet may impact different microbial colonization and the function of microbiomes. An understanding and control of diet-microbiome-host relations will result in personal diet routes, shaped by one’s physiological conditions and adapted to distinct nutritional needs. This aims at addressing the big open concern, which is the effect of diet on the gut microbiome and the growing impact of the gut microbiome on health [75-77].
Integration into clinical practice
Armed with microbiome information, healthcare providers can craft exceptionally personalized nutritional advice. Precision nutrition emerges as a powerful tool in modifying the course of gastrointestinal disorders, among which irritable bowel syndrome (IBS) is one of the most common diseases. Tailored dietary strategies based on the unique gut microbiota composition, which can enhance the effectiveness of dietary interventions, have been suggested as the future direction in managing IBS [1].
Microbes inhabit various parts of the human body, including the gastrointestinal (GI) tract; approximately 95% of the microbes live in the GI tract. The gut microbiota increases the bioavailability of food ingredients, produces metabolites that are transported to different parts of the body, and is involved in the metabolic transformation of food ingredients. Maintenance of gut microbiota homeostasis is closely related to human health, and an imbalance is observed in several diseases such as obesity, inflammatory bowel disease, and non-alcoholic fatty liver disease. On the other side, the gut microbiota is a target of some pharmacological treatments. Intrinsic factors such as gender, ethnicity, and age, and extrinsic factors such as diet, hygiene, antibiotic use, and mode of delivery modulate the composition of the gut microbiome. Diet is a major environmental factor that shapes gut microbial communities [54].
The dietary components can influence the gut microbial composition within a few weeks. Nutrigenomics is a study area that focuses on the relationship between diet and genetics and how their interactions provide positive and negative effects on human health. Inter-individual variation in gene sequences can influence the bioavailability and metabolism of specific nutrients; this concept has provided a scientific basis for personalized nutrition. Personalized nutrition strategies aim to design tailored dietary recommendations that vary interpersonally, avoiding a “one-size-fits-all” approach. Recent research has shown that intestinal microbes can provide new opportunities for personalized nutrition. The bidirectional relationship between diet and gut microbiota is closely maintained and influences the host’s health status. This review will help us to understand the potential of gut microbiome-based personalized diets [25]. With the rapid development of sequencing technology, the relationship between diet, genes, and the gut microbiome has been elucidated, and offers the possibility of providing personalized, tailored interventions on a huge scale.
- Shang Z, Pai L, Patil S. Unveiling the dynamics of gut microbial interactions: a review of dietary impact and precision nutrition in gastrointestinal health. Front Nutr. 2024;11:1395664. Available from: https://doi.org/10.3389/fnut.2024.1395664
- Abeltino A, Hatem D, Serantoni C, Riente A, De Giulio MM, De Spirito M, et al. Unraveling the gut microbiota: implications for precision nutrition and personalized medicine. Nutrients. 2024;16(22):3806. Available from: https://www.mdpi.com/2072-6643/16/22/3806
- Bianchetti G, De Maio F, Abeltino A, Serantoni C, Riente A, Santarelli G, … Maulucci G. Unraveling the gut microbiome–diet connection: exploring the impact of digital precision and personalized nutrition on microbiota composition and host physiology. Nutrients. 2023;15(18):3931. Available from: https://www.mdpi.com/2072-6643/15/18/3931
- Sisk‑Hackworth L, Kelley ST, Thackray VG. Sex, puberty, and the gut microbiome. Reproduction. 2023;165(2):R61–R74. Available from: https://rep.bioscientifica.com/view/journals/rep/165/2/REP-22-0303.xml
- Barreto HC, Gordo I. Intrahost evolution of the gut microbiota. Nat Rev Microbiol. 2023;21(9):590-603. Available from: https://doi.org/10.1038/s41579-023-00890-6
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. Available from: https://www.mdpi.com/2076-2607/7/1/14
- Miyauchi E, Shimokawa C, Steimle A, Desai MS, Ohno H. The impact of the gut microbiome on extra‑intestinal autoimmune diseases. Nat Rev Immunol. 2023;23(1):9-23. Available from: https://www.nature.com/articles/s41577-022-00727-y
- Di Tommaso N, Santopaolo F, Gasbarrini A, Ponziani FR. The gut–vascular barrier as a new protagonist in intestinal and extraintestinal diseases. Int J Mol Sci. 2023;24(2):1470. Available from: https://www.mdpi.com/1422-0067/24/2/1470
- Hong M, Cheng L, Liu Y, Wu Z, Zhang P, Zhang X. Mechanisms underlying the interaction between chronic neurological disorders and microbial metabolites via tea polyphenols therapeutics. Front Microbiol. 2022;13:823902. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2022.823902
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351938/
- Sarkar A, Yoo JY, Dutra VO, Morgan KH, Groer M. The association between early-life gut microbiota and long-term health and diseases. J Clin Med. 2021;10(3):459. Available from: https://www.mdpi.com/2077-0383/10/3/459
- Turroni F, Milani C, Ventura M, van Sinderen D. The human gut microbiota during the initial stages of life: insights from bifidobacteria. Curr Opin Biotechnol. 2022;73:81-87. Available from: https://doi.org/10.1016/j.copbio.2021.07.012
- Martino C, Dilmore AH, Burcham ZM, Metcalf JL, Jeste D, Knight R. Microbiota succession throughout life from the cradle to the grave. Nat Rev Microbiol. 2022;20(12):707-720. Available from: https://doi.org/10.1038/s41579-022-00768-z
- Guo J, Ren C, Han X, Huang W, You Y, Zhan J. Role of IgA in the early-life establishment of the gut microbiota and immunity: implications for constructing a healthy start. Gut Microbes. 2021;13(1):1–21. Available from: https://doi.org/10.1080/19490976.2021.1908101
- Olsson LM, Boulund F, Nilsson S, Khan MT, Gummesson A, Fagerberg L, et al. Dynamics of the normal gut microbiota: a longitudinal one‑year population study in Sweden. Cell Host Microbe. 2022;30(5):726–739. Available from: https://doi.org/10.1016/j.chom.2022.03.002
- Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GCY, Ng SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post‑acute COVID‑19 syndrome. Gut. 2022;71(3):544–552. Available from: https://doi.org/10.1136/gutjnl-2021-325989
- Luo Y, Ren W, Smidt H, Wright ADG, Yu B, Schyns G, et al. Dynamic distribution of gut microbiota in pigs at different growth stages: composition and contribution. Microbiol Spectr. 2022;10(3):e00688-21. Available from: https://doi.org/10.1128/spectrum.00688-21
- Aldars‑García L, Marin AC, Chaparro M, Gisbert JP. The interplay between the immune system and microbiota in inflammatory bowel disease: a narrative review. Int J Mol Sci. 2021;22(6):3076. Available from: https://doi.org/10.3390/ijms22063076
- Ullah H, Arbab S, Tian Y, Liu CQ, Chen Y, Qijie L, et al. The gut microbiota–brain axis in neurological disorders. Front Neurosci. 2023;17:1225875. Available from: https://doi.org/10.3389/fnins.2023.1225875
- Ghosh TS, Shanahan F, O’Toole PW. Toward an improved definition of a healthy microbiome for healthy aging. Nat Aging. 2022;2:253–266. Available from: https://doi.org/10.1038/s43587-022-00306-9
- Hoskinson C, Dai DL, Del Bel KL, Becker AB, Moraes TJ, Mandhane PJ, et al. Delayed gut microbiota maturation in the first year of life is a hallmark of pediatric allergic disease. Nat Commun. 2023;14(1):4785. Available from: https://doi.org/10.1038/s41467-023-40336-4
- Ranheim Sveen T, Hannula SE, Bahram M. Microbial regulation of feedbacks to ecosystem change. Trends Microbiol. 2024;32(1):68-78. Available from: https://doi.org/10.1016/j.tim.2023.06.006
- Pan X, Raaijmakers JM, Carrión VJ. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023; 31(9):959-97. Available from: https://doi.org/10.1016/j.tim.2023.03.018
- Vernocchi P, Del Chierico F, Putignani L. Gut microbiota metabolism and interaction with food components. Metabolites. 2020; 21(10):3688. Available from: https://doi.org/10.3390/ijms21103688
- Sarfraz MH, Shahid A, Asghar S, Aslam B, Ashfaq UA, Raza H, et al. Personalized nutrition, microbiota, and metabolism: a triad for eudaimonia. Metabolites. 2022; 9:1038830. Available from: https://doi.org/10.3389/fmolb.2022.1038830
- Arnolds L, Lozupone CA. Striking a balance with help from our little friends – how the gut microbiota contributes to immune homeostasis. Semin Immunol. 2016;28(5):517–524. Available from: https://pubmed.ncbi.nlm.nih.gov/27698623/
- Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022;18(7):and page(s). Available from: https://doi.org/10.1038/s41582-022-00697-8
- Ramanan D, Pratama A, Zhu Y, Venezia O, Sassone‑Corsi M, Chowdhary K, et al. Regulatory T cells in the face of the intestinal microbiota. Nat Rev Immunol. 2023;23(11):749-762. Available from: https://doi.org/10.1038/s41577-023-00890-w
- Suchiita A, Gupta N, Nandi K, Sonkar S, Chandra L. Harmony within: unravelling the microbiome–immune system symbiosis for health. Adv Gut Microbiome Res. 2025;1:9927379. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1155/agm3/9927379
- Khor B, Snow M, Herrman E, Ray N, Mansukhani K, Patel KA, et al. Interconnections between the oral and gut microbiomes: reversal of microbial dysbiosis and the balance between systemic health and disease. Microorganisms. 2021;9(3):496. Available from: https://doi.org/10.3390/microorganisms9030496
- Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms, and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717-726. Available from: https://doi.org/10.1038/s41575-022-00634-6
- Kho YZ, Lal KS. The human gut microbiome – a potential controller of wellness and disease. 2018;9:1835. Available from: https://doi.org/10.3389/fmicb.2018.01835
- Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, et al. Defining the roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23(7):499-515. Available from: https://doi.org/10.1038/s41580-022-00456-z
- Andrabi SM, Sharma NS, Karan A, Shahriar SS, Cordon B, Ma B, Xie J. Nitric oxide: physiological functions, delivery, and biomedical applications. Adv Sci. 2023;10(30):2303259. Available from: https://doi.org/10.1002/advs.202303259
- Infante‑Villamil S, Huerlimann R, Jerry DR. Microbiome diversity and dysbiosis in aquaculture. Rev Aquac. 2021;13(2):1077-1096. Available from: https://ui.adsabs.harvard.edu/abs/2021RvAq...13.1077I/abstract
- Wang F, Liu Q, Wu H, Tang T, Zhao T, Li Z. The dysbiosis gut microbiota induces the alternation of metabolism and imbalance of Th17/Treg in OSA patients. Arch Microbiol. 2022; 204(4):217. Available from: https://doi.org/10.1007/s00203-022-02825-w
- Haneishi Y, Furuya Y, Hasegawa M, Picarelli A, Rossi M, Miyamoto J. Inflammatory bowel diseases and gut microbiota. Int J Mol Sci. 2023;24(4):3817. Available from: https://doi.org/10.3390/ijms24043817
- Santana PT, Rosas SLB, Ribeiro BE, Marinho Y, de Souza HS. Dysbiosis in inflammatory bowel disease: pathogenic role and potential therapeutic targets. Int J Mol Sci. 2022;23(7):3464. Available from: https://doi.org/10.3390/ijms23073464
- Pisani A, Rausch P, Bang C, Ellul S, Tabone T, Marantidis Cordina C, et al. Dysbiosis in the gut microbiota in patients with inflammatory bowel disease during remission. Microbiol Spectr. 2022;10(3):e00616-22. Available from: https://doi.org/10.1128/spectrum.00616-22
- Ballini A, Scacco S, Boccellino M, Santacroce L, Arrigoni R. Microbiota and obesity: where are we now? 2020;9(12):415. Available from: https://doi.org/10.3390/biology9120415
- Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and disease. Signal Transduct Target Ther. 2022;7(1):135. Available from: https://doi.org/10.1038/s41392-022-00974-4
- Liu J, Tan Y, Cheng H, Zhang D, Feng W, Peng C. Functions of gut microbiota metabolites, current status, and future perspectives. Aging Dis. 2022;13(4):1106. Available from: https://doi.org/10.14336/ad.2022.0104
- Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, et al. Human gut microbiota in health and disease: unveiling the relationship. Front Microbiol. 2022;13:999001. Available from: https://doi.org/10.3389/fmicb.2022.999001
- Christovich A, Luo X. Gut microbiota, leaky gut, and autoimmune diseases. 2022; 13:946248. Available from: https://doi.org/10.3389/fimmu.2022.946248
- Mousa WK, Chehadeh F, Husband S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front Immunol. 2022;13: 906258. Available from: https://doi.org/10.3389/fimmu.2022.906258
- Topi S, Bottalico L, Charitos IA, Colella M, Di Domenico M, Palmirotta R, Santacroce L. Biomolecular mechanisms of autoimmune diseases and their relationship with the resident microbiota: friend or foe? Pathophysiology. 2022;29(3):507-536. Available from: https://doi.org/10.3390/pathophysiology29030041
- Wang X, Yuan W, Yang C, Wang Z, Zhang J, Xu D, Sun W. Emerging role of gut microbiota in autoimmune diseases. Front Immunol. 2024;15:1365554. Available from: https://doi.org/10.3389/fimmu.2024.1365554
- Wu HJ, Zegarra‑Ruiz D, Diehl GE. Intestinal microbes in autoimmune and inflammatory disease. 2020;11:597966. Available from: https://doi.org/10.3389/fimmu.2020.597966
- Halper‑Stromberg A, Jabri B. Maladaptive consequences of inflammatory events shape individual immune identity. Nat Immunol. 2022; 23(12):1675-1686. Available from: https://doi.org/10.1038/s41590-022-01342-8
- Yau C, Danska JS. Cracking the type 1 diabetes code: genes, microbes, immunity, and the early life environment. Immunol Rev. 2024; 325(1):23-45. Available from: https://doi.org/10.1111/imr.13362
- Sun J, Chen F, Wu G. Potential effects of gut microbiota on host cancers: focus on immunity, DNA damage, cellular pathways, and anticancer therapy. 2023; 17(10):1535-1551. Available from: https://doi.org/10.1038/s41396-023-01483-0
- Xu Z, Lv Z, Chen F, Zhang Y, Xu Z, Huo J, Lu A. Dysbiosis of human tumor microbiome and aberrant residence of Actinomyces in tumor‑associated fibroblasts in young‑onset colorectal cancer. Front Immunol. 2022;13:1008975. Available from: https://doi.org/10.3389/fimmu.2022.1008975
- Lin Z, Mao D, Jin C, Wang J, Lai Y, Zhang Y, Sheng L. The gut microbiota correlates with the disease characteristics and immune status of patients with untreated diffuse large B‑cell lymphoma. Front Immunol. 2023;14:1105293. Available from: https://doi.org/10.3389/fimmu.2023.1105293
- Song EJ, Shin JH. Personalized diets based on the gut microbiome as a target for health maintenance: from current evidence to future possibilities. 2022;32(12):1497-1505. Available from: https://doi.org/10.4014/jmb.2209.09050
- Kuntz MK, Gilbert JA. Introducing the microbiome into precision medicine. 2016; 38(1):81-91. Available from: https://doi.org/10.1016/j.tips.2016.10.001
- Nogal B, Blumberg JB, Blander G, Jorge M. Gut microbiota–informed precision nutrition in the generally healthy individual: are we there yet? 2021; 5(9):nzab107. Available from: https://doi.org/10.1093/cdn/nzab107
- Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. 2012; 2012:872716. Available from: https://doi.org/10.1155/2012/872716
- Schmidt TS, Li SS, Maistrenko OM, Akanni W, Coelho LP, Dolai S, et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat Med. 2022;28(9):1902‑1912. Available from: https://doi.org/10.1038/s41591-022-01913-0
- Shtossel O, Turjeman S, Riumin A, Goldberg MR, Elizur A, Bekor Y, … Louzoun Y. Recipient‑independent, high‑accuracy FMT‑response prediction and optimization in mice and humans. Microbiome. 2023;11(1):181. Available from: https://doi.org/10.1186/s40168-023-01623-w
- Lin A, Jiang A, Huang L, Li Y, Zhang C, Zhu L,et al. From chaos to order: optimizing fecal microbiota transplantation for enhanced immune checkpoint inhibitors efficacy. Gut Microbes. 2025;17(1):2452277. Available from: https://doi.org/10.1080/19490976.2025.2452277
- Tian H, Wang X, Fang Z, Li L, Wu C, Bi D, et al. Fecal microbiota transplantation in clinical practice: present controversies and prospects. HLife. 2024;2(6):269‑283. Available from: https://doi.org/10.1016/j.hlife.2024.01.006
- Brüssow H. Problems with the concept of gut microbiota dysbiosis. 2019;13(2):423-434. Available from: https://doi.org/10.1111/1751-7915.13479
- Foster‑Nyarko E, Pallen MJ. The microbial ecology of Escherichia coli in the vertebrate gut. FEMS Microbiol Rev. 2022;46(3):fuac008. Available from: https://doi.org/10.1093/femsre/fuac008
- Sharon I, Quijada NM, Pasolli E, Fabbrini M, Vitali F, Agamennone V, … Turroni S. The core human microbiome: does it exist and how can we find it? a critical review of the concept. Nutrients. 2022;14(14):2872. Available from: https://doi.org/10.3390/nu14142872
- Abdullah MM, Vazquez‑Vidal I, Baer DJ, House JD, Jones PJ, Desmarchelier C. Common genetic variations involved in the inter-individual variability of circulating cholesterol concentrations in response to diets: a narrative review of recent evidence. Nutrients. 2021;13(2):695. Available from: https://doi.org/10.3390/nu13020695
- Lange L, Berg G, Cernava T, Champomier‑Vergès MC, Charles T, Cocolin L, … Sessitsch A. Microbiome ethics, guiding principles for microbiome research, use, and knowledge management. 2022;17(1):50. Available from: https://doi.org/10.1186/s40793-022-00444-y
- Sainz T, Pignataro V, Bonifazi D, Ravera S, Mellado MJ, Pérez‑Martínez A, Escudero A, Ceci A, Calvo C. Human microbiome in children, at the crossroad of social determinants of health and personalized medicine. 2021;8(12):1191. Available from: https://doi.org/10.3390/children8121191
- Wei L, Singh R, Ro S, Ghoshal UC. Gut microbiota dysbiosis in functional gastrointestinal disorders: underpinning the symptoms and pathophysiology. JGH Open. 2021;5(9):976-987. Available from: https://doi.org/10.1002/jgh3.12528
- Stolfi C, Maresca C, Monteleone G, Laudisi F. Implications of intestinal barrier dysfunction in gut dysbiosis and diseases. Biomedicines. 2022;10(2):289. Available from: https://doi.org/10.3390/biomedicines10020289
- Li XY, Meng L, Shen L, Ji HF. Regulation of gut microbiota by vitamin C, vitamin E, and β‑carotene. Food Res Int. 2023;169:112749. Available from: https://doi.org/10.1016/j.foodres.2023.112749
- Hossain KS, Amarasena S, Mayengbam S. B vitamins and their roles in gut health. Microorganisms. 2022;10(6):1168. Available from: https://doi.org/10.3390/microorganisms10061168
- Brun P. The profiles of dysbiotic microbial communities. 2019;5(1):87-101. Available from: https://doi.org/10.3934/microbiol.2019.1.87
- Ruiz‑Malagón AJ, Rodríguez‑Sojo MJ, Redondo E, Rodríguez‑Cabezas ME, Gálvez J, Rodríguez‑Nogales A. Systematic review: the gut microbiota as a link between colorectal cancer and obesity. Obes Rev. 2025;26(4):e13872. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/obr.13872
- Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;153:113415. Available from: https://doi.org/10.1016/j.biopha.2022.112678
- Caballero‑Flores G, Pickard JM, Núñez G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nat Rev Microbiol. 2023;21(6):347‑360. Available from: https://doi.org/10.1038/s41579-022-00833-7
- Ross FC, Patangia D, Grimaud G, Lavelle A, Dempsey EM, Ross RP, Stanton C. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol. 2024;22(11):671‑686. Available from: https://doi.org/10.1038/s41579-024-01068-4
- Davis EC, Castagna VP, Sela DA, Hillard MA, Lindberg S, Mantis NJ, … Järvinen KM. Gut microbiome and breast-feeding: implications for early immune development. J Allergy Clin Immunol. 2022;150(3):523‑534. Available from: https://doi.org/10.1016/j.jaci.2022.07.014