More Information
Submitted: December 19, 2023 | Approved: June 23, 2025 | Published: June 24, 2025
How to cite this article: Al-Saboonchi AA, Al-Kaisse GA, Al-Timimi IH, Jalal SS, Marashi HA. Effect of Different Levels of Salinity on Growth and Lipid Content in Freshwater Microalgae. Arch Case Rep. 2025; 9(7): 207-212. Available from:
https://dx.doi.org/10.29328/journal.acr.1001148
DOI: 10.29328/journal.acr.1001148
Copyright license: © 2025 Al-Saboonchi AA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Salinity; Growth; Culture; Microalgae lipid; Cyanobacteria
Effect of Different Levels of Salinity on Growth and Lipid Content in Freshwater Microalgae
Azhar A Al-Saboonchi1*, Ghalib A Al-Kaisse2, Ihsan H Al-Timimi2, Sarah S Jalal2 and Hasanean A Marashi3
1Al Kunooze University College, Iraq
2National University for Science and Technology, Iraq
3General Company of South Electric Distribution, Basra, Iraq
*Address for Correspondence: Azhar A Al-Saboonchi, Al Kunooze University College, Iraq, Email; [email protected]
Salinity imposes stress on various living organisms like microalgae, the study focuses on the effect of salinity concentrations on cell growth and lipid content for four isolated freshwater microalgae (Chlorella vulgaris, Scenedesmus sp., Nitzschia palea, and Anabaena sp.). These selected algae were cultured in different treatments of salinity (0.5, 1.5, 1.5, and 2.5 gm/l). The results show that changes in salinity have significant effects on growth and lipid content, the growth tends to decline as salinity increases. Lipid content was different among studying algae depending on the range of tolerance, the amount of lipids increased when salinity increased too.
Energy is essential for development, and the use of fossil fuels as energy is unstable due to the accumulation of greenhouse gases in the environment, for that many ideas have been considered to develop environmentally friendly alternatives to fossil fuels [1]. According to some analysts, crude oil will be depleted before 2060 Rodolfi, et al. 2009.
Biofuels are fuels that are derived from organic or biological components Osman, et al. 2021, and are classified into three-generation biofuels [2], Chowdhury, et al. 2019.
The first production of biofuels was made from feedstock, but these resources for energy production were stopped due to rising food prices and terrestrial plants are limited by land availability [3]. Then were depended on animal waste, which was digested for the production of biogas. Later the production of biofuels depended on algae (macro and microalgae) [4], especially based on microalgae, these ways will be the available solution to the problems associated with first and second-generation biofuels; microalgae are regarded as a viable feedstock for various bioproducts and bioenergy carriers [5].
Much literature demonstrates algal-based biofuels economic and environmental impacts Efroymson, et al. 2021; El-Shimi, et al. 2018 [5]. Chisti [4] mentioned that microalgae biofuel has the potential to completely displace petro-diesel and meet the global demand for fuels. For that microalgae can be used for biofuel production is widely regarded Schenk, et al. 2008. Some microalgae have achieved 50% to 60% of dry weight as lipids Sheehan, et al. 1998. The single-cell structure of most microalgae has the potential to autocomplete conventional crops [4], due to fast growth and high oil production [4]. The production of lipids by microalgae (10 – 20) is times greater per unit area compared to agricultural products Chaisutyakorn, et al. 2017.
Algae fuels are limited by the availability of carbon dioxide, nutrients, and water [6]. Chisti [4] mentioned that microalgae double their biomass within 24 hours.
Microalgae have a low amount of lignin making them less resistant to degradation [7]. There are advantages of using microalgae since it have a short life cycle, easy to cultivate in the laboratory [8]. Many species of microalgae can produce a high quantity of carbohydrates [9].
The aim of the present study is to investigate the ability of some isolated freshwater microalgae to produce lipids at different levels of salinity and determine the lipid concentrations in isolated microalgae.
Freshwater samples of algae were collected from different stations at Shatt Al-Arab using a phytoplankton net (mesh size 0.2 µm). Samples were transported to the sterile container (150 ml). In laboratory samples incubated under controlled conditions for algae growth (280 µE/ m2/s; 16:8 light: dark photoperiod and 25 ± 2 °C).
Two techniques were used for algal isolation (dilution and streaking on plate agar). The serial dilution method was followed by using 12 test tubes, the first test tube contained 1 ml of algal sample and nutrient solution, then shook and 1 ml from it was transported to the second test tube and so on until the final test tube [10]. During the process of dilution, examination of each dilution with the microscope to isolate one species of algae.
The second method is streaking on plate agar containing media solidified by 15% agar-agar and sterilized by autoclave then was poured into Petri-dishes and left to solidify, sterile loop was used for streaking straight line, petri-dishes were kept in a cooled illuminated incubator with light intensity 268 µE/m2/s, with 25 ± 2 °C, photoperiod was 16:8 light: dark for 12 days [11]. This method was repeated until uni-algal cultures were obtained.
Chlorella vulgaris Bejerinck; Senedesmus sp.; Nitzschia palea (Ktz.) W. Smith and Anabaena sp. were isolated and identified according to Bellinger and Sigee [12], Desikachary [13] and Prescott [10].
A small quantity of the confirmed uni algal was transferred into a 250 ml flask containing Chu-10 medium solution, these flask was incubated under standard cultivation conditions for 12 days to get appropriate growth [10]. These cultures were renewed weekly. Every day the cultures were shaken by hand.
These selected algae were undergone in different treatments of salinity, four concentrations of NaCl were used (0.5, 1.0, 1.5, 2.5) g/l to observe the viability of algae to the growth and lipid production under the circumstances of stimulus. For that 32 flasks were prepared to study the effect of salinity, each flask (250 ml) contained 90 ml of chu-10, then 10 ml of the old culture of different isolated algae were added to each flask, and then the flasks were incubated under standard cultivation conditions (light intensity 268 µE/m2/s with 16:8 light: dark period, and 25 ± 2 °C). Isolated algae were incubated for 14 days. Samples were collected to measure growth (by measuring OD) and lipid production, from microalgae culture at different levels of salinity.
Total lipids were determined gravimetrically by a modified Bligh and Dyer (1959) method Takagi, et al. 2006, 100 g of dried algae were used for extraction, algae were harvested by means of filtration, filter paper containing the microalgae were freeze-dried overnight for lipid extraction the following day.
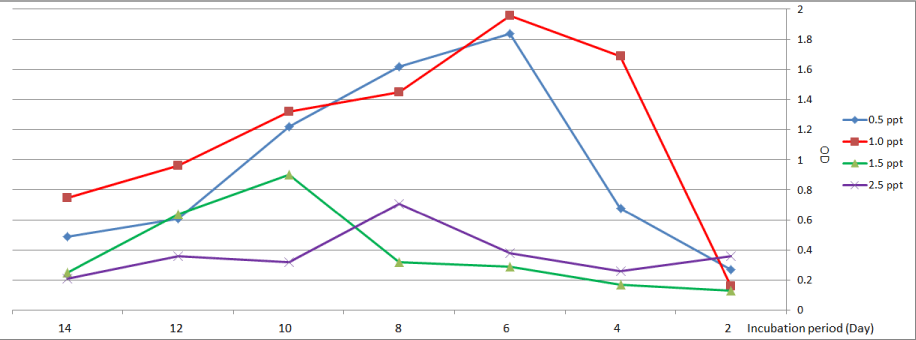
In the case of Chlorella vulgaris at treatment with (0.5 and 1.0) ppt, the highest growth was observed on the sixth day (1.84 and 1.96) respectively. On another hand growth of C. vulgaris decreased with increased salinity, at media with (1.5 and 2.5) ppt highest growth was recorded on the tenth day and the eighth day respectively (Figure 1).
Figure 1: Growth curve of Chlorella vulgaris at different levels of salinity.
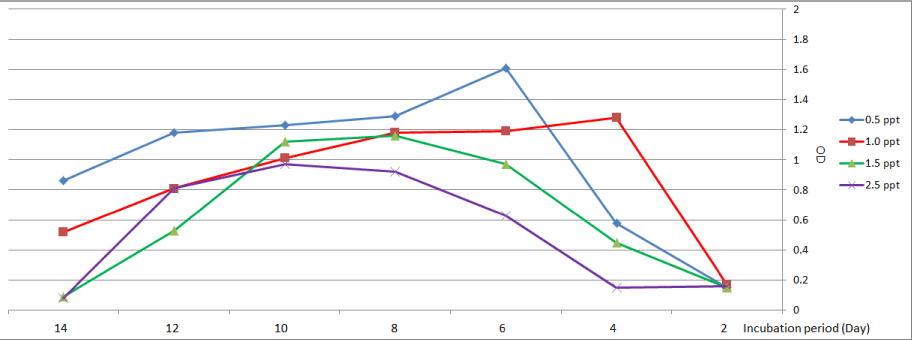
Scenedesmus sp. showed the highest growth at treatment with (0.5 and 1.0) on the sixth and the fourth day respectively, while at culturing in media (1.5 and 2.5) ppt highest growth was recorded on the eighth day and the tenth day respectively (Figure 2).
Figure 2: Growth curve of Scenedesmus sp. at different levels of salinity.
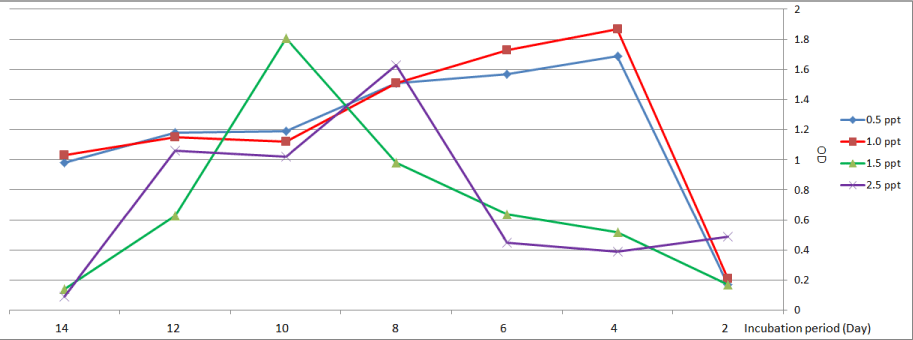
Figure 3 shows the highest growth for Nitzschia palea was observed on the fourth day at treatment with (0.5 and 1.0) ppt, at treatment with a high level of salinity (1.5 and 2.5) ppt, the highest growth was recorded at the tenth day and eighth day respectively.
Figure 3: Growth curve of Nitzschia palea at different levels of salinity.
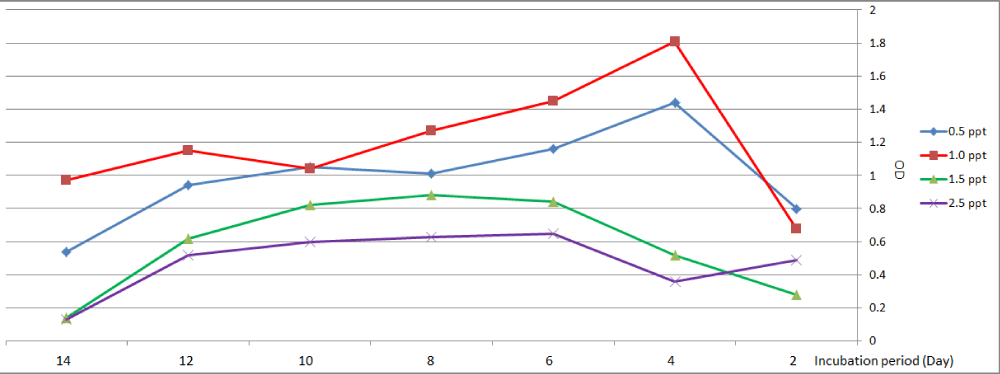
The cyanophycea Anabaena sp. showing maximum growth at salinity (0.5 and 1.0) ppt were observed on the fourth day. At salinity 1.5 ppt maximum growth on the eighth day, but in the case of treatment with 2.5 ppt highest growth was recorded on the sixth day (Figure 4).
Figure 4: Growth curve of Anabaena sp. at different levels of salinity.
Microalgae differ in adaptability to salinity and other stress conditions. The results showed that lipid production by microalgae was stimulated at different levels of salinity.
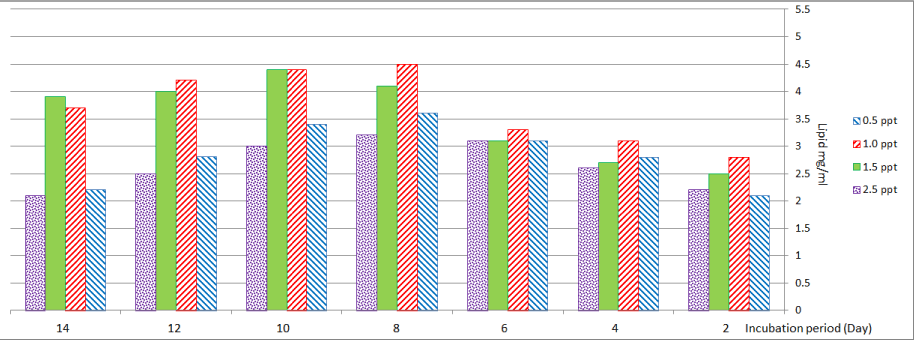
Lipid production by C. vulgaris was stimulated by lower salinity (0.5 and 1.0) ppt and reached maximum values on the eighth day (Figure 5).
Figure 5: Lipid content of Chlorella vulgaris at different levels of salinity.
At higher salinity (1.5) ppt maximum value of lipid content was observed on the tenth day. At salinity (2.5) ppt lipid content was lower than that observed at lower salinity.
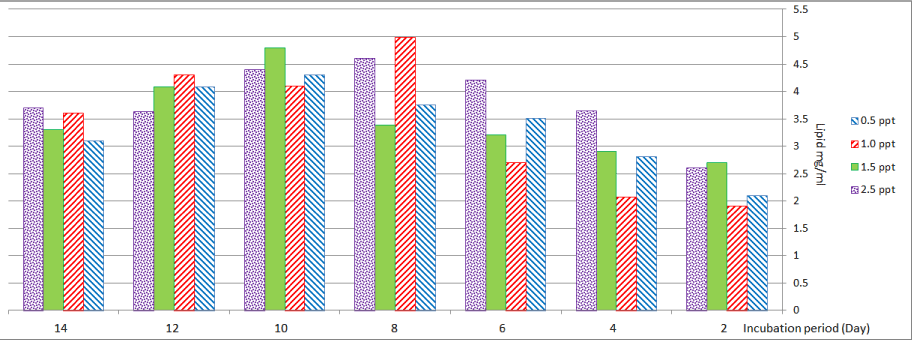
As shown in Figure 6, Scenedesmus sp. that culturing in media with (0.5 and 1.0) ppt showed maximum values for lipid production (4.3 and 4.98) mg/ml on the tenth day and the eighth day respectively. In the case of culturing in media with (1.5) ppt, the highest value for lipid (4.8 mg/ml) was recorded on the tenth day, while at media with (2.5) ppt maximum value (4.6) mg/ml was recorded on the eighth day.
Figure 6: Lipid content of Scenedesmus sp. at different levels of salinity.
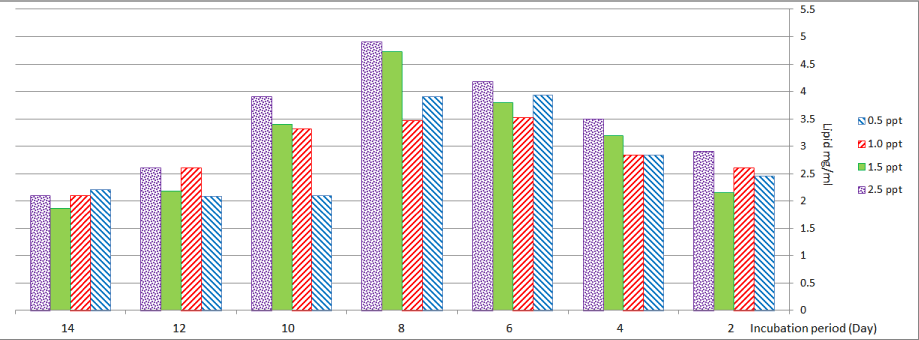
Nitzschia palea showed maximum lipid content (3.39 and 3.52) mg/ml at treatment with (0.5 and 1.0) ppt respectively.
Amounts of lipids were (4.72 and 4.90) mg/ml at treatment with (1.5 and 2.5) ppt on the eighth day respectively (Figure 7).
Figure 7: Lipid content of Nitzschia palea at different levels of salinity.
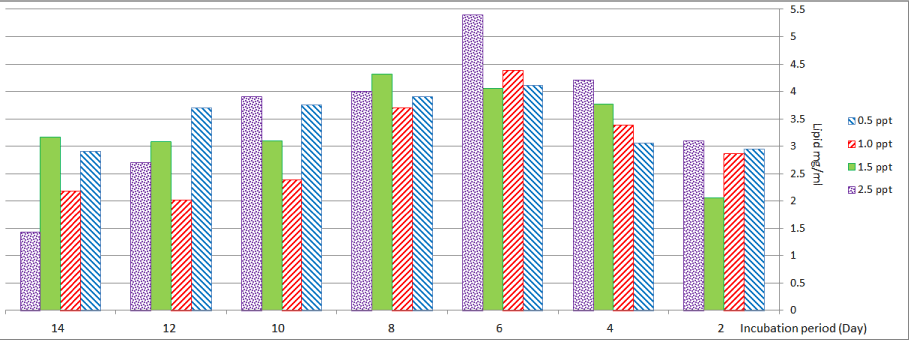
Figure 8 shows the effect of different levels of salinity on Anabaena sp. at treatment with salinity (0.5 and 1.0) ppt, lipid production was (4.11 and 4.39) mg/l on the sixth day. Maximum lipid production occurred at a salinity of (1.5) ppt on the eighth day, but in the case of salinity (2.5) ppt maximum lipid production (5.4) mg/ml was recorded on the sixth day.
Figure 8: Lipid content of Anabaena sp. at different levels of salinity.
Schenk, et al. (2008) mentioned that microalgae are used widely for the production of fuel. And salinity is considered a very important factor affecting growth and metabolic activities [14], also salinity plays an important role in lipid production.
Mus, et al. [15] showed that Chlorella sp. biomass was affected by salinity, and the biomass decreased with increasing salinity, the same result was observed at the growth of C. vulgaris in medium with (2.5) ppt. Monika, et al. [16] mentioned that Chlorella sp. growing under salt supplemented medium will produce a high amount of oil for biodiesel production, the possible explanation for lipid enhancement is the metabolic pathway of microalgae toward lipid accumulation by synthesis of acetyl CoA. Takagiet, et al. 2006, studied the effect of salt on lipid production in Dunaliella tertiolecta, a 7% increase in lipid content when salinity concentration doubled.
In the case of Scenedesmus sp. highest amount of lipid production with salinity (1.5) ppt, Kaewkannetra, et al. 2012 reported that Scenedesmus has salt tolerance.
A change in the total and intracellular composition of lipids in response to environmental salinity has been reported by various authors Xu and Beardoll, 1997; Abid, et al. 2008, they mentioned that increasing in salinity of microalgae cultures will produce more oil, the same was observed in this study.
Accumulation of lipids in microalgae occurs during environmental stress, increasing salt concentration affects the rate of respiration and permeability of cell membranes [17]. The amount of saturated fatty acid in algae decreases as the salinity increases [18].
Brown, et al. 1997 mentioned that diatoms contain more lipids than other algae, the present study found that Nitzschia palea contains lipids at different levels of salinity and the highest amount was recorded at treatment with (2.5) ppt, the same was reported by Griffiths and Harrison (2009), during their studying on diatom Amphora coffeaeformis which showing the ability to increase its lipid production at higher salinity.
Cyanobacteria have several ranges of salinity, but the information in the literature is rather complicated about the range of tolerance Vonshak and Torzillo, 2004; Bilanovic, et al. 2009. Salinity affects the growth of microalgae acting directly on the osmo-regulatory mechanism of the cell. As the increase or decrease of salinity, generated stress inside the algae, total lipid content increased as storage reserve energy material till favorable conditions arise, results from this study show lipid content of Anabaena sp. increases with increasing salinity [19-26].
This study found that salinity significantly impacts the growth and lipid content of freshwater microalgae (Chlorella vulgaris, Scenedesmus sp., Nitzschia palea, Anabaena sp.). While higher salinity generally inhibited growth, it promoted lipid accumulation, particularly in Nitzschia palea and Anabaena sp. at 2.5 ppt. These findings suggest the potential of certain microalgae for biofuel production in saline environments and underscore the importance of optimizing salinity as a cultivation parameter. Further research into long-term stability and underlying biochemical mechanisms is recommended.
- Klass LD. Biomass for renewable energy, fuels and chemicals. New York: Academic Press. 1998;1–2. Available from: https://www.scirp.org/reference/referencespapers?referenceid=1964504
- Koh LP, Ghazoul J. Biofuels, biodiversity and people: understanding the conflicts and finding opportunities. Biol Conserv. 2008;141:2450–2460. Available from: https://doi.org/10.1016/j.biocon.2008.08.005
- Huesemann MH. Can advances in science and technology prevent global warming? A critical review of limitations and challenges. Mitig Adapt Strateg Glob Change. 2006;11:539–577. Available from: https://www.scirp.org/reference/referencespapers?referenceid=708096
- Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294–306. Available from: https://doi.org/10.1016/j.biotechadv.2007.02.001
- Ermis H, Altinbas M. Effect of salinity on mixed microalgae grown in anaerobic liquid digestant. Water Environ J. 2020;34(S1):820–830. Available from: https://ui.adsabs.harvard.edu/abs/2020WaEnJ..34S.820E/abstract
- Pate R, Klise G, Wu B. Resource demand implications for US algae production scale up. Appl Energy. 2011;88:3377–3388. Available from: https://doi.org/10.1016/j.apenergy.2011.04.023
- Wargacki AJ, Leonard E, Win MN, Regitsky D, Santos C, Kim P. An engineered microbial platform for direct biofuel production from brown microalgae. Science. 2012;335(6066):305–313. Available from: https://doi.org/10.1126/science.1214547
- Wayman C. Handbook of bioethanol production and utilization. Washington (DC): Taylor and Francis; 1996. Available from: https://doi.org/10.1201/9780203752456
- Eshaq FS, Ali MN, Mold MK. Production of bioethanol from next generation feedstock algae Spirogyra species. Int J Eng Sci Technol. 2011;3:1749–1755. Available from: https://www.researchgate.net/publication/50406997_Production_of_Bioethanol_from_next_generation_feed-stock_alga_Spirogyra_species
- Prescott GW. Algae of the Western Great Area. 2nd ed. Koenigstein: Otto Koeltz Science Publishers; 1982.
- Sinigalliano CD, Winshell J, Guerrero M, Scorzetti G, Fell J, Eaton R, Brand L, Rein KS. Viable cell sorting of dinoflagellates by multiparametric flow cytometry. Phycologia. 2009;48:249–257. Available from: https://doi.org/10.2216/08-51.1
- Bellinger EC, Sigee DC. Algae as bio-indicators. In: Freshwater algae: identification and use as bio-indicators. Chichester (UK): John Wiley and Sons Ltd.; 2010;1–150.
- Desikachary TV. Cyanophyta. New Delhi: Indian Council of Agricultural Research; 1959;686. Available from: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1693451
- Carrieri D, Momot D, Brasg IA, Ananyev G, Lenz OB, Bryant DA. Boosting fermentation rates and product yield with sodium stress: application to renewable fuel production by Cyanobacteria. Appl Environ Microbiol. 2010;76(19):6455–6462. Available from: https://doi.org/10.1128/aem.00975-10
- Mus F, Dubini A, Seibert M, Posewitz MC, Grossman AR. Anaerobic adaptation in Chlamydomonas reinhardtii: anoxic gene expression, hydrogenase induction and metabolic pathways. J Biol Chem. 2007;282:25475–25486. Available from: https://doi.org/10.1074/jbc.m701415200
- Monika PR, Trishnamoni G, Nikunj S. Effect of salinity, pH and light intensity on growth and lipid production of microalgae for bioenergy application. J Biol Sci. 2015;:260–267. Available from: https://thescipub.com/abstract/ojbsci.2015.260.267
- Sudhir P. Effect of salt stress on basic processes of photosynthesis. Photosynthetica. 2004;42:481–486. Available from: https://link.springer.com/article/10.1007/S11099-005-0001-6
- Kirrolia A, Bishnoi RN, Singh N. Salinity as a factor affecting the physiological and biochemical traits of Scenedesmus sp. J Algal Biomass Util. 2011;2:328–341. Available from: https://storage.unitedwebnetwork.com/files/521/65823ac7d7dbbe44224c9d0679c3e2d7.pdf
- Jawad AM. Interaction between cyanobacteria and other micro-organisms [dissertation]. Liverpool (UK): University of Liverpool; 1982. Available from: https://livrepository.liverpool.ac.uk/3175724/1/331923.pdf
- Khan SA, Rashmi M, Hussain SP, Banerjee UC. Prospects of biodiesel production from microalgae in India. Renew Sustain Energy Rev. 2009;13(9):2361–2372. Available from: https://doi.org/10.1016/j.rser.2009.04.005
- Hussain S, Salleh A. Biodiesel fuel production from algae as renewable energy. Am J Biochem Biotechnol. 2008;4(3):250–254. Available from: https://thescipub.com/pdf/ajbbsp.2008.250.254.pdf
- Carere CR, Sparling R, Cicek N, Levin DB. Third generation biofuels via direct cellulose fermentation. Int J Mol Sci. 2008;9:1342–1360. Available from: https://doi.org/10.3390/ijms9071342
- Phuka MM, Chutia RS, Konwar BK, Kataki R. Microalgae Chlorella as a potential bio-energy feedstock. Appl Energy. 2011;88(10):3307–3312. Available from: https://ideas.repec.org/a/eee/appene/v88y2011i10p3307-3312.html
- Borges FC. Proposal of a conceptual model of a decentralized biorefinery [dissertation]. Porto Alegre (RS): Universidade Federal do Rio Grande do Sul; 2010. Portuguese. Available from: https://lume.ufrgs.br/bitstream/handle/10183/24714/000744737.pdf;sequence=1
- AOAC International. Official methods of analysis. 16th ed. Gaithersburg (MD): AOAC International; 1995.
- Mulumba N, Farag IH. Tubular photobioreactor for microalgae biodiesel production. Int J Eng Technol. 2012;4(2):703–709. Available from: https://www.researchgate.net/publication/259866896_Tubular_Photobioreactor_for_Microalgae_Biodiesel_Production