More Information
Submitted: May 30, 2025 | Approved: June 10, 2025 | Published: June 11, 2025
How to cite this article: Bonetti M, Frigerio M, Fontana A, Maffezzoni F, Miglio S. Incidental finding of a Rare Congenital Subcutaneous Lumbar Arteriovenous Fistula Prior to Infiltrative Treatment for L5-S1 Disc Herniation: A Case Study. Arch Case Rep. 2025; 9(6): 188-192. Available from:
https://dx.doi.org/10.29328/journal.acr.1001144
DOI: 10.29328/journal.acr.1001144
Copyright license: © 2025 Bonetti M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Arteriovenous fistula; Congenital vascular malformation; Lumbar region; Incidental finding; Magnetic resonance imaging; Sciatica
Incidental finding of a Rare Congenital Subcutaneous Lumbar Arteriovenous Fistula Prior to Infiltrative Treatment for L5-S1 Disc Herniation
M Bonetti1*, M Frigerio1, A Fontana2, F Maffezzoni3 and S Miglio3
1Department of Neuroradiology, Clinical Institute City of Brescia, Brescia, Italy
2Department of Radiology, Villa Barbarano Nursing Home, Via Spiaggia D’oro 21 25087 Barbarano, Brescia, Italy
3Oberdan Specialist Outpatient Clinic, Guglielmo Oberdan Street 126, 25128 Brescia, Italy
*Address for Correspondence: M Bonetti, Department of Neuroradiology, Clinical Institute City of Brescia, Brescia, Italy, Email: [email protected]
Arteriovenous fistulas (AVFs) are abnormal connections between an artery and a vein, characterized by a low-resistance shunt that bypasses the normal capillary bed. Their etiology can be either congenital or acquired; congenital forms, although rare, may remain clinically silent for years and be discovered incidentally during imaging performed for unrelated symptoms. We report the case of a 47-year-old woman with right-sided lumbosciatica due to an L5-S1 disc herniation, who underwent pre-infiltrative lumbosacral magnetic resonance imaging (MRI). In addition to the herniated disc with nerve root compression, MRI incidentally revealed a superficial vascular malformation in the thoracolumbar subcutaneous tissues, compatible with a congenital arteriovenous fistula. To our knowledge, no analogous cases have been previously reported in the literature. This rare finding highlights the importance of thorough pre-procedural imaging evaluation, especially when planning invasive treatments near the affected anatomical region.
Arteriovenous Fistulas (AVFs) are abnormal communications between an artery and a vein that bypass the capillary bed, resulting in a low-resistance, high-flow shunt. This hemodynamic deviation can impair tissue perfusion and, in more severe cases, lead to local hyperflow phenomena, chronic venous congestion, or, in extreme conditions, high-output cardiac failure [1-3].
From an etiological perspective, AVFs are classified as either congenital or acquired. The congenital forms, which are rarer, stem from vascular development anomalies during embryogenesis and may occur in various anatomical locations (brain, lungs, liver, kidneys, skin), presenting with variable degrees of severity. In contrast, acquired AVFs are more common and typically result from penetrating trauma, bone fractures, surgical procedures, percutaneous biopsies, or iatrogenic maneuvers such as central or arterial catheter placement [4,5]. Although many traumatic AVFs become symptomatic early, delayed presentations years after the triggering event are not uncommon [6].
Paraspinal Arteriovenous Fistulas (PAVFs) represent a rare subset of spinal AVFs and may be located outside the vertebral canal, along the paravertebral venous pathways. According to the Stuttgart classification proposed by Wendl, et al. [6], these shunts are categorized as isolated (congenital, acquired, or traumatic) or associated with metameric/genetic syndromes, and further subdivided based on their venous drainage pattern (intraspinal or exclusively extraspinal). Isolated congenital forms are often asymptomatic and diagnosed incidentally but may become clinically relevant due to spinal venous congestion or spinal cord compression caused by ectatic veins.
From an anatomopathophysiological standpoint, PAVFs originate from shunts between segmental arteries (or their branches) and paraspinal venous plexuses, with potential reflux into the epidural or perimedullary venous system. The lack of venous valves within the spinal venous network facilitates pathological reflux in the presence of chronic venous hypertension, contributing to neurological symptoms.
The diagnostic work-up is multimodal, beginning with ultrasound and color-Doppler imaging, which are useful in detecting abnormal flow patterns and characterizing the lesion morphology. Magnetic Resonance Imaging (MRI) provides a detailed assessment of the anatomical extent and relationship with adjacent structures. In complex cases or in candidates for interventional treatment, selective angiography may be indicated.
Management of AVFs requires a personalized and multimodal approach, potentially involving advanced imaging, endovascular interventions, or surgery, depending on the shunt’s type and location.
Spinal Arteriovenous Malformations (AVMs) constitute a complex chapter of neurovascular pathology, with significant clinical and prognostic implications. Within this heterogeneous group, it is crucial to distinguish Spinal Dural Arteriovenous Fistulas (SDAVFs) from the rarer non-dural forms, including epidural, perimedullary, paraspinal, and subcutaneous fistulas, each characterized by specific anatomical location, flow dynamics, and clinical impact.
Differential classification of spinal arteriovenous fistulas
Spinal AVMs represent a complex and varied subset of neurovascular conditions. It is essential to differentiate SDAVFs from the much rarer non-dural spinal AVFs, such as epidural, perimedullary, paraspinal, and subcutaneous fistulas, each with distinct anatomical and hemodynamic features and clinical implications.
Spinal Dural Arteriovenous Fistulas (SDAVFs): SDAVFs are the most common type of spinal vascular malformation, accounting for over 70% of symptomatic spinal AVFs [7-9]. These are typically acquired lesions, located within the dural sleeve of the nerve root, caused by an abnormal connection between a radiculomeningeal artery and a radicular vein. Venous drainage occurs into the perimedullary venous plexus, leading to progressive venous hypertension and congestive myelopathy, often presenting with slowly progressive motor and sensory deficits that may go unrecognized in the early stages [10-18].
Non-dural spinal arteriovenous fistulas: Epidural, Perimedullary, Paraspinal, and Subcutaneous: Non-dural spinal AVFs represent a much rarer and heterogeneous group. These fistulas are located outside the dura mater, with distinct venous drainage patterns. They are frequently congenital, though they may also arise acquired after trauma, surgery, or vascular developmental anomalies.
Specifically:
- Spinal epidural fistulas: originate in the epidural space and typically drain into the paraspinal or epidural venous systems. Symptoms may resemble those of SDAVFs, though the hemodynamics differ significantly [18-20].
- Perimedullary fistulas: often seen in younger individuals, involve the anterior spinal arteries and drain directly into the intramedullary venous system. Clinical presentation may include subarachnoid hemorrhage or acute myelopathy [21].
- Paraspinal and subcutaneous fistulas: such as the one observed in our case, are exceptionally rare. In these cases, the arteriovenous shunt is located within the superficial soft tissues, between muscular or segmental arteries and subcutaneous veins. These lesions are often asymptomatic and found incidentally during imaging studies. However, they acquire clinical relevance when invasive procedures are planned in the same region. In such scenarios, the main risk is hemorrhagic complications resulting from direct trauma or iatrogenic manipulation [22].
Anatomical considerations on spinal cord and subcutaneous vascularization: The posterior spinal arteries are two slender vascular structures that run along the dorsal surface of the spinal cord. They typically originate from the vertebral arteries, although they may also arise from collateral branches of the vertebrobasilar system. These arteries play a critical role in supplying the posterior one-third of the spinal cord, including the posterior nerve roots and the posterior median septa.
It is essential to emphasize that the posterior spinal arteries do not give off branches to the skin or subcutaneous tissue. The subcutaneous vascularization is instead ensured by cutaneous and subcutaneous branches originating from distinct arterial districts, depending on the anatomical region:
- Thoracic and dorsal region: branches of the posterior intercostal arteries;
- Lumbar region: branches of the lumbar arteries;
- Sacral region: branches of the lateral sacral arteries or from the pelvic arterial system;
- Cervical and nuchal region: transverse cervical arteries and occipital arteries.
These vessels traverse the muscular layers to reach the superficial tissues, providing a blood supply that is independent of the posterior spinal arterial system. The distinction between the spinal cord and cutaneous vascular territories is of critical neuroanatomical and clinical importance, particularly in the interpretation of neurological symptoms and in the planning of surgical or interventional procedures involving the vertebro-medullary structures.
We report the case of Mrs. V.S., a 47-year-old woman, who presented with right-sided lumbosciatica persisting for several weeks, with pain radiating along the right S1 radicular distribution. Based on the clinical presentation and suspicion of a disc-root conflict, the referring physician requested a lumbosacral MRI.
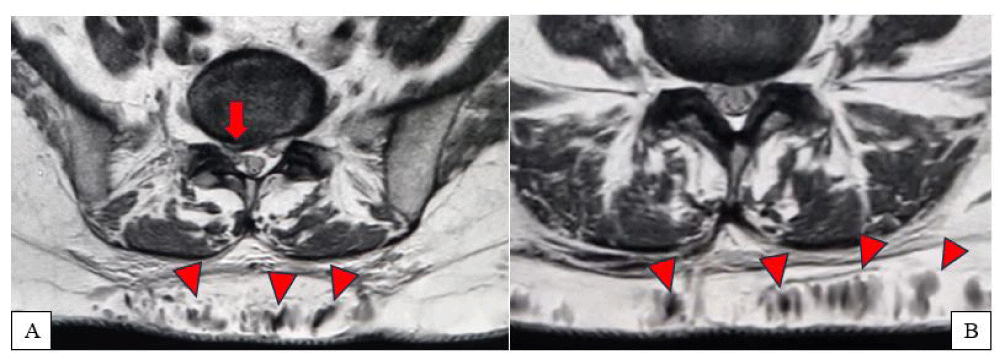
The imaging revealed a right paramedian L5-S1 disc herniation, compressing the ipsilateral S1 nerve root, a finding consistent with the patient’s radicular pain symptoms.
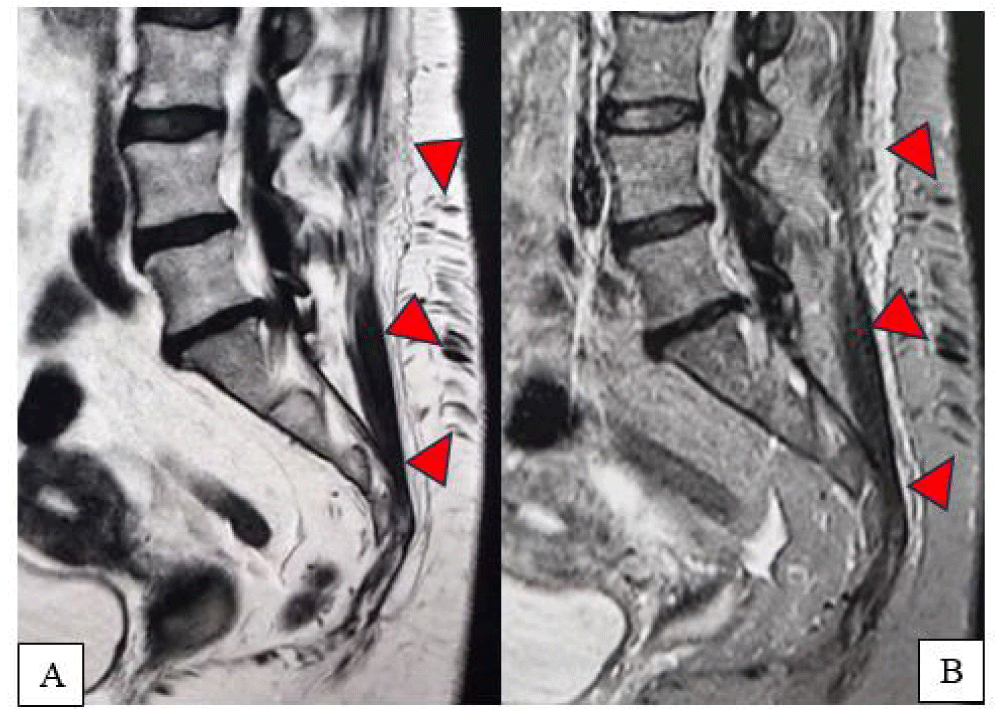
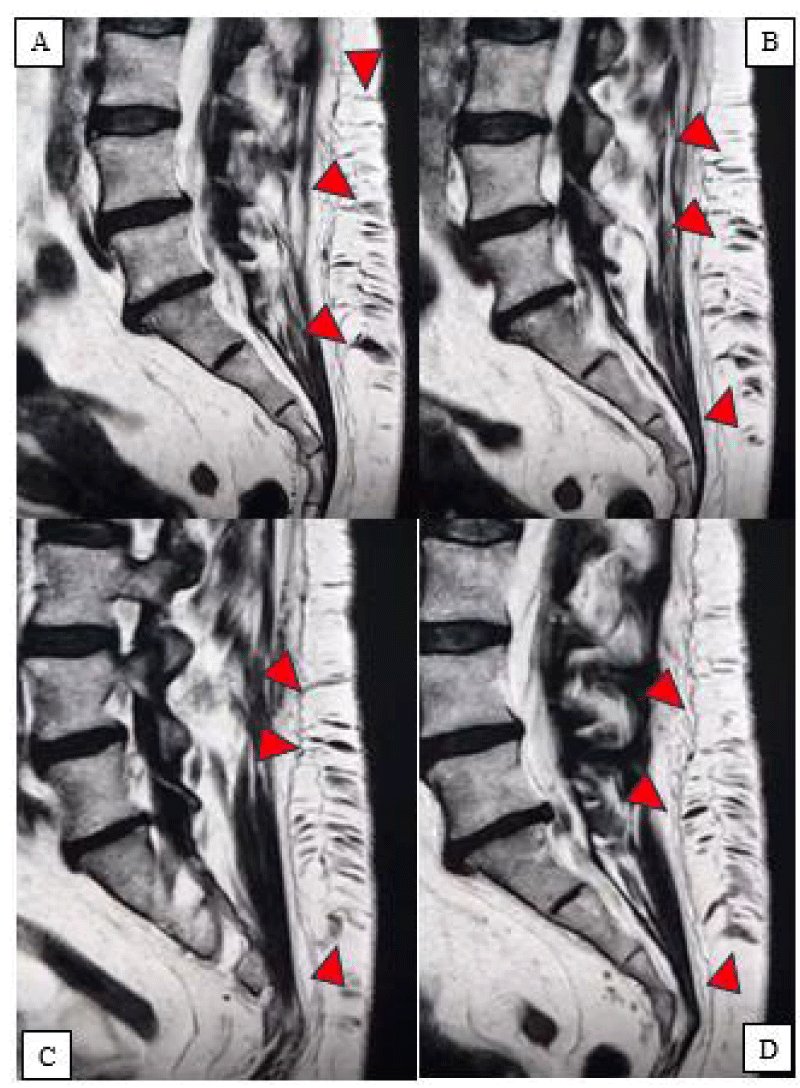
Incidentally, the MRI also showed the presence of a subcutaneous vascular malformation in the lumbar region, compatible with a congenital arteriovenous fistula, extending from L3 to S3. The lesion appeared entirely asymptomatic, with no signs of compression on adjacent neural structures. The patient’s history was negative for prior trauma at that level (Figures 1-3).
Figure 1(A,B): Axial MRI scan showing a right paramedian subligamentous L5-S1 disc herniation with compressive effect on the right S1 nerve root (arrow). Concurrently, dilated subcutaneous veins at the S1 level are observed (arrowheads).
Figure 2(A,B): Sagittal lumbosacral MRI, T2-weighted (A) and T2 Fat-Sat (B) sequences: evidence of a subcutaneous lumbar arteriovenous fistula, extending through the superficial soft tissues from the L3 to S3 region, with marked dilation of the subcutaneous veins (arrowheads).
Figure 3(A-D): Sagittal lumbosacral MRI, Subcutaneous lumbar arteriovenous fistula (arrowheads).
The lesion appeared completely asymptomatic and was unrelated to the patient’s reported pain, consistent with a rare subcutaneous lumbar arteriovenous fistula. Given the potential risk of hemorrhagic complications, the planned infiltrative procedure was suspended, and a vascular diagnostic work-up was initiated to further characterize the morphological and functional aspects of the shunt.
The vascular finding was interpreted as a potential risk factor for the scheduled procedure. Consequently, we deemed it appropriate to postpone the intervention and refer the patient back to her primary care physician for specialist angiological or neurovascular evaluation.
In light of the absence of symptoms directly attributable to the vascular malformation and the patient’s overall clinical stability, a conservative “wait-and-see” approach was agreed upon, involving clinical monitoring and follow-up imaging. The lumbosciatic symptoms were managed through pharmacological therapy combined with a targeted physiotherapy program, with any future invasive treatments postponed until after appropriate specialist assessment.
The authors chose to present this clinical case due to the exceptional nature of the finding: a completely asymptomatic lumbar subcutaneous arteriovenous fistula, discovered incidentally during an MRI scan performed for lumbosciatica. To date, similar cases involving arteriovenous fistulas located in the subcutaneous tissue of the lumbosacral region have not been described in the scientific literature, making this case particularly relevant from both anatomopathological and clinical perspectives.
In daily practice, the most frequently observed spinal arteriovenous fistulas are dural fistulas, whose clinical presentation, prognostic implications, and therapeutic strategies differ significantly. Within this context, the accurate identification and classification of the lesion played a crucial role in guiding the clinical decision-making process.
Although asymptomatic, the vascular finding was interpreted as an “anatomical warning”, indicating potential risk in the setting of invasive procedures involving the lumbar region. Specifically, the possibility of accidental puncture of the dilated venous channels or the arterial feeders of the fistula represented a complication to be avoided. For this reason, the initially planned infiltrative procedure was suspended.
A conservative “wait-and-see” approach was adopted, and the patient was referred back to her primary care physician for specialist evaluation and follow-up. This choice was supported by two main factors: the absence of symptoms directly related to the fistula and the favorable natural course generally associated with L5-S1 disc herniation, which often resolves or stabilizes over time. Notably, the patient did not present with any neurovascular deficits, but rather complained of isolated right-sided lumbosciatica.
Although there were no formal contraindications to the infiltrative procedure, a cautious approach was favored, postponing any therapeutic decision to a later stage, should symptoms persist or worsen.
This case highlights the importance of a thorough diagnostic assessment during the initial patient work-up. Awareness of individual vascular variants, although rare, may play a key role in preventing complications and in adapting treatment strategies toward a more personalized and safe approach.
- Konner K, Nonnast-Daniel B, Ritz E. The arteriovenous fistula. J Am Soc Nephrol. 2003;14(6):1669-80. Available from: https://doi.org/10.1097/01.asn.0000069219.88168.39
- Quencer KB, Arici M. Arteriovenous fistulas and their characteristic sites of stenosis. Am J Roentgenol. 2015;205(4):726-34. Available from: https://doi.org/10.2214/ajr.15.14650
- Jayroe H, Foley K. Arteriovenous Fistula. 2022 Nov 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://pubmed.ncbi.nlm.nih.gov/32644639/
- Gonzalez-Araiza G, Haddad L, Patel S, Karageorgiou J. Percutaneous Embolization of a Postsurgical Prostatic Artery Pseudoaneurysm and Arteriovenous Fistula. J Vasc Interv Radiol. 2019;30(2):269-71. Available from: https://doi.org/10.1016/j.jvir.2018.11.011
- Faughnan ME, Lui YW, Wirth JA, Pugash RA, Redelmeier DA, Hyland RH, White RI. Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest. 2000;117(1):31-8. Available from: https://doi.org/10.1378/chest.117.1.31
- Antoniou GA, Lazarides MK, Georgiadis GS, Sfyroeras GS, Nikolopoulos ES, Giannoukas AD. Lower-extremity arteriovenous access for haemodialysis: a systematic review. Eur J Vasc Endovasc Surg. 2009;38(3):365-72. Available from: https://doi.org/10.1016/j.ejvs.2009.06.003
- Nepal S, Annamaraju P. Coronary Arteriovenous Fistula. 2022. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554618/
- Wendl CM, Aguilar Pérez M, Felber S, Stroszczynski C, Bäzner H, Henkes H. Paraspinal arteriovenous fistula: Stuttgart classification based on experience and a review of the literature. Br J Radiol. 2018;91(1088):20170337. Available from: https://doi.org/10.1259/bjr.20170337
- Brinjikji W, Colombo E, Cloft HJ, Lanzino G. Clinical and Imaging Characteristics of Spinal Dural Arteriovenous Fistulas and Spinal Epidural Arteriovenous Fistulas. Neurosurgery. 2021;88(3):666-73. Available from: https://doi.org/10.1093/neuros/nyaa492
- Krishnan D, Viswanathan S, Rose N, Benjamin HSN, Ong AM, Hiew FL. Clinical heterogeneity of low flow spinal arteriovenous fistulas; a case series. BMC Neurol. 2021;21:1-8. Available from: https://doi.org/10.1186/s12883-021-02394-3
- Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. Am J Neuroradiol. 2009;30(4):639-48. Available from: https://doi.org/10.3174/ajnr.a1485
- Peng Y, Ren Y, Hou J, Zhang C, He M, Huang B, et al. Clinical outcomes and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas: a retrospective study of 118 patients. Sci Rep. 2023;13(1):18266. Available from: https://doi.org/10.1038/s41598-023-45599-x
- Fox S, Hnenny L, Ahmed U, Meguro K, Kelly ME. Spinal dural arteriovenous fistula: a case series and review of imaging findings. Spinal Cord Ser Cases. 2017;3(1):1-6. Available from: https://doi.org/10.1038/scsandc.2017.24
- Jeng Y, Chen DY, Hsu HL, Huang YL, Chen CJ, Tseng YC. Spinal dural arteriovenous fistula: imaging features and its mimics. Korean J Radiol. 2015;16(5):1119-31. Available from: https://doi.org/10.3348/kjr.2015.16.5.1119
- Filis A, Romualdo SM, Engellandt K, El-Battrawy I, Podlesek D, Juratli TA, et al. Diagnostic, clinical management, and outcomes in patients with spinal dural arteriovenous fistula. Front Surg. 2024;11:1374321. Available from: https://doi.org/10.3389/fsurg.2024.1374321
- Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129(Pt 12):3150-64. Available from: https://doi.org/10.1093/brain/awl220
- Brinjikji W, Yin R, Nasr DM, Lanzino G. Spinal epidural arteriovenous fistulas. J Neurointerv Surg. 2016;8(12):1305-10. Available from: https://doi.org/10.1136/neurintsurg-2015-012181
- Da Ros V, Picchi E, Ferrazzoli V, Schirinzi T, Sabuzi F, Grillo P, et al. Spinal vascular lesions: anatomy, imaging techniques and treatment. Eur J Radiol Open. 2021;8:100369. Available from: https://doi.org/10.1016/j.ejro.2021.100369
- Nasr DM, Brinjikji W, Clarke MJ, Lanzino G. Clinical presentation and treatment outcomes of spinal epidural arteriovenous fistulas. J Neurosurg Spine. 2017;26(5):613-20. Available from: https://doi.org/10.3171/2016.9.spine16618
- Brinjikji W, Colombo E, Cloft HJ, Lanzino G. Clinical and imaging characteristics of spinal dural arteriovenous fistulas and spinal epidural arteriovenous fistulas. Neurosurgery. 2021;88(3):666-73. Available from: https://doi.org/10.1093/neuros/nyaa492
- Demirci O, Celayir A. Prenatal diagnosis and treatment of intrahepatic arteriovenous fistulas: case reports and the literature review. J Matern Fetal Neonatal Med. 2022;35(5):837-45. Available from: https://doi.org/10.1080/14767058.2020.1731466
- Lenck S, Nicholson P, Tymianski R, Hilditch C, Nouet A, Patel K, et al. Spinal and paraspinal arteriovenous lesions: uncovering vascular pathology of the spine. Stroke. 2019;50(8):2259-69. Available from: https://doi.org/10.1161/strokeaha.118.012783